NLRP3-IN-2
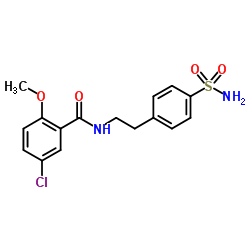
NLRP3-IN-2 structure
|
Common Name | NLRP3-IN-2 | ||
|---|---|---|---|---|
| CAS Number | 16673-34-0 | Molecular Weight | 368.835 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C16H17ClN2O4S | Melting Point | 209-214 °C | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of NLRP3-IN-2NLRP3-IN-2, an intermediate substrate in the synthesis of glyburide, inhibits the formation of the NLRP3 inflammasome in cardiomyocytes and limits the infarct size following myocardial ischemia/reperfusion in the mouse, without affecting glucose metabolism[1]. |
| Name | 4-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzene Sulfonamide |
|---|---|
| Synonym | More Synonyms |
| Description | NLRP3-IN-2, an intermediate substrate in the synthesis of glyburide, inhibits the formation of the NLRP3 inflammasome in cardiomyocytes and limits the infarct size following myocardial ischemia/reperfusion in the mouse, without affecting glucose metabolism[1]. |
|---|---|
| Related Catalog | |
| In Vivo | NLRP3-IN-2 is well tolerated with no effects on the glucose levels in vivo[1]. NLRP3-IN-2 (100 mg/kg) treatment in a model of AMI due to ischemia+reperfusion significantly inhibits the activity of inflammasome (caspase-1) in the heart by 90% (P<0.01) and reduced infarct size, measured at pathology (by >40%, P<0.01) and with troponin I levels (by >70%, P<0.01) [1]. Animal Model: Experimental acute myocardial infarction (AMI) model in mice[1]. Dosage: 100 mg/kg. Administration: Intraperitoneal administration 30 minutes prior to surgery, then every 6 hours for 3 additional doses. Result: Led to a significant >90% reduction in caspase-1 activity (reflective of the formation of an active inflammasome) in the heart tissue measured 24 hours after ischemia. Led to a significant reduction in the infarct size measured with TTC (>40% reduction) or troponin I levels (>70% reduction) when compared with vehicle alone. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 209-214 °C |
| Molecular Formula | C16H17ClN2O4S |
| Molecular Weight | 368.835 |
| Exact Mass | 368.059753 |
| PSA | 106.87000 |
| LogP | 1.74 |
| Index of Refraction | 1.598 |
| Storage condition | Refrigerator |
| Hazard Codes | T |
|---|---|
| Risk Phrases | R23/24/25 |
| Safety Phrases | S26-S36-S36/37-S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2935009090 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse.
J. Cardiovasc. Pharmacol. 63(4) , 316-22, (2014) The formation of the NLRP3 inflammasome in the heart during acute myocardial infarction amplifies the inflammatory response and mediates further damage. Glyburide has NLRP3 inhibitory activity in vitr... |
|
|
Trendelenburg G.
J. Cereb. Blood Flow Metab. , (2014)
|
|
|
Reply to Letter Regarding Article," NLRP3 Inflammasome as a Therapeutic Target in Myocardial Infarction. Takahashi M.
Int. Heart J. 55(4) , 380, (2014)
|
| 5-chloro-2-methoxy-N-[2-(4-sulphamoylphenyl)ethyl]benzamide |
| Benzamide, N-[2-[4-(aminosulfonyl)phenyl]ethyl]-5-chloro-2-methoxy- |
| 5-Chloro-2-methoxy-N-[2-(4-sulfamoylphenyl)ethyl]benzamide |
| 4-[2-(5-chloro-2-methoxy-benzamide)ethyl]-benzenesulphonamide |
| EINECS 240-722-5 |
| 5-Chloro-2-methoxy-N-(4-sulfamoylphenethyl)benzamide |
| MFCD00193756 |
 CAS#:29568-33-0
CAS#:29568-33-0 CAS#:35303-76-5
CAS#:35303-76-5![Ethyl 4-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzene Sulfonamide Carbamate structure](https://image.chemsrc.com/caspic/424/14511-59-2.png) CAS#:14511-59-2
CAS#:14511-59-2
