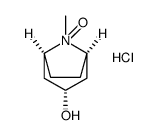
Tropine
Tropine structure
|
Common Name | Tropine | ||
|---|---|---|---|---|
| CAS Number | 120-29-6 | Molecular Weight | 141.211 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 233.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H15NO | Melting Point | 50-60 °C(lit.) | |
| MSDS | USA | Flash Point | 112.5±14.5 °C | |
Use of TropineTropine is a secondary metabolite of Solanaceae plants, is an anticholinergic agent[1]. Tropine is a common intermediate in the synthesis of a variety of bioactive alkaloids, including hyoscyamine and scopolamine[2]. |
| Name | tropine |
|---|---|
| Synonym | More Synonyms |
| Description | Tropine is a secondary metabolite of Solanaceae plants, is an anticholinergic agent[1]. Tropine is a common intermediate in the synthesis of a variety of bioactive alkaloids, including hyoscyamine and scopolamine[2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 233.0±0.0 °C at 760 mmHg |
| Melting Point | 50-60 °C(lit.) |
| Molecular Formula | C8H15NO |
| Molecular Weight | 141.211 |
| Flash Point | 112.5±14.5 °C |
| Exact Mass | 141.115356 |
| PSA | 23.47000 |
| LogP | -0.10 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.526 |
| Storage condition | 2-8°C |
| Water Solubility | 100 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R20/22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| RTECS | YM3875000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Tropine dehydrogenase: purification, some properties and an evaluation of its role in the bacterial metabolism of tropine.
Biochem. J. 307 ( Pt 2) , 603-8, (1995) Tropine dehydrogenase was induced by growth of Pseudomonas AT3 on atropine, tropine or tropinone. It was NADP(+)-dependent and gave no activity with NAD+. The enzyme was very unstable but a rapid puri... |
|
|
The metabolism of atropine in man.
J. Pharm. Pharmacol. 38(10) , 781-4, (1986) A metabolic pattern of atropine in man, based on the detection of radiolabelled products in urine by high performance liquid chromatography after administration of [3H]atropine sulphate to a normal vo... |
|
|
Determination of nitrogen-15 isotope fractionation in tropine: evaluation of extraction protocols for isotope ratio measurement by isotope ratio mass spectrometry.
Rapid Commun. Mass Spectrom. 23(24) , 4031-7, (2009) N-Demethylation of tropine is an important step in the degradation of this compound and related metabolites. With the purpose of understanding the reaction mechanism(s) involved, it is desirable to me... |
| (1R,5S)-8-Methyl-8-azabicyclo[3.2.1]octan-3-ol |
| 8-Methyl-8-azabicyclo[3.2.1]octan-3-ol |
| Tropine |
| endo-Tropanol |
| TROPAN-3ALPHA-OL |
| 3alpha-Tropanol |
| 8-Azabicyclo[3.2.1]octan-3-ol, 8-methyl- |
| EINECS 204-384-2 |
| MFCD00005551 |
| 8-Azabicyclo[3.2.1]octan-3-ol, 8-methyl-, (1R,5S)- |
| endo-8-Methyl-8-azabicyclo[3.2.1]octan-3-ol |
| tropinol |
| 3-TROPANOL |
| TROPANOL |
 CAS#:1234788-77-2
CAS#:1234788-77-2 CAS#:50-00-0
CAS#:50-00-0![8-Aza-bicyclo[3.2.1]octan-3-ol HCl Structure](https://image.chemsrc.com/caspic/103/538-09-0.png) CAS#:538-09-0
CAS#:538-09-0 CAS#:115522-58-2
CAS#:115522-58-2 CAS#:115522-57-1
CAS#:115522-57-1 CAS#:115522-45-7
CAS#:115522-45-7 CAS#:51-55-8
CAS#:51-55-8![(1R,3r,5S)-3-hydroxy-8-isopropyl-8-methyl-8-azabicyclo[3.2.1]octan-8-ium iodide Structure](https://image.chemsrc.com/caspic/426/93713-44-1.png) CAS#:93713-44-1
CAS#:93713-44-1 CAS#:532-24-1
CAS#:532-24-1![8-Methyl-8-azabicyclo[3.2.1]oct-3-yl 2H-indazole-3-carboxylate structure](https://image.chemsrc.com/caspic/463/107007-94-3.png) CAS#:107007-94-3
CAS#:107007-94-3![8-Azabicyclo[3.2.1]octane-8-carboxaldehyde, 3-hydroxy-, endo- (9CI) structure](https://image.chemsrc.com/caspic/181/50626-97-6.png) CAS#:50626-97-6
CAS#:50626-97-6 CAS#:40796-97-2
CAS#:40796-97-2![endo-8-Methyl-8-azabicyclo[3.2.1]octan-3-yl 2-hydroxy-2,2-diphenylacetate structure](https://image.chemsrc.com/caspic/273/3736-36-5.png) CAS#:3736-36-5
CAS#:3736-36-5![ENDO-8-METHYL-8-AZABICYCLO[3.2.1]OCTAN-3-YL METHANESULFONATE structure](https://image.chemsrc.com/caspic/157/35130-97-3.png) CAS#:35130-97-3
CAS#:35130-97-3![1,2-bis(3-α-hydroxy-8-azabicyclo[3,2,1]oct-8-yl)ethane structure](https://image.chemsrc.com/caspic/274/80830-76-8.png) CAS#:80830-76-8
CAS#:80830-76-8 CAS#:464-72-2
CAS#:464-72-2![8-(2-hydroxy-2,2-diphenylethyl)-3α-hydroxy-8-azabicyclo[3.2.1]octane structure](https://image.chemsrc.com/caspic/312/80830-75-7.png) CAS#:80830-75-7
CAS#:80830-75-7
