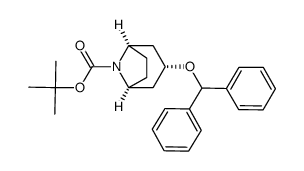
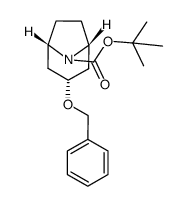
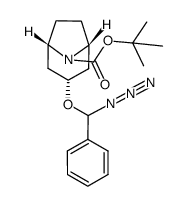
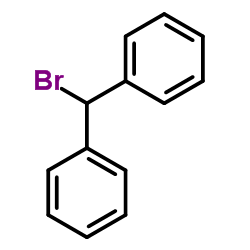
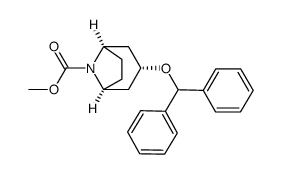
86-13-5
| Name | benzatropine |
|---|---|
| Synonyms |
Benaztropine
benzotropine exo-Benztropine tropine benzohydryl ether |
| Description | Benztropine mesylate is an orally active centrally acting anticholinergic agent that can be used for Parkinson's disease research[1]. Benztropine mesylate is an anti-histamine agent and a dopamine re-uptake inhibitor. Benztropine mesylate is also a human D2 dopamine receptor allosteric antagonist. Benztropine mesylate also has anti-CSCs (cancer stem cells) effects[2]. |
|---|---|
| Related Catalog | |
| Target |
5 μM (MDA-MB-231) |
| In Vitro | Benztropine mesylate (0.1-10 μM; 72 hours) treatment inhibits the cell growth of MDA-MB-231 cells with an IC50 of ~5 μM. In MDA-MB-231 cells and 4T1-luc2 cells, Benztropine mesylate reduces the size as well as the number of mammospheres significantly in a dose-dependent manne[1]. Benztropine mesylate inhibits functions of cancer stem cells (CSCs) via the acetylcholine receptors, dopamine transporters/receptors, and/or histamine receptors[1]. Benztropine mesylate induces the differentiation of oligodendrocytes through M1 and M3 muscarinic receptors and enhanced re-myelination[1]. Cell Viability Assay[1] Cell Line: MDA-MB-231 Concentration: 0, 0.1, 0.625, 1.25, 2.5, 5, 10 μM Incubation Time: 72 hours Result: Inhibited cells growth of MDA-MB-231 with IC50 ~5.0 μM. Cell Viability Assay[1] Cell Line: MDA-MB-231 Concentration: 0, 1, 2, 5 μM Incubation Time: 4-6 days Result: Suppressed mammosphere formation and self-renewal capacities of BCSCs in a dose-dependent manner in vitro. |
| In Vivo | Benztropine mesylate (5 μM, 4 weeks) inhibits tumor-initiating potential in a 4T1 mouse model[1]. Animal Model: Balb/c mice bearing 4T1 breast tumors[1] Dosage: 1.5 mg/kg Administration: Injection; 3 weeks Result: Reduced the tumor size and weight significantly without body weight changing. |
| References |
| Density | 1.11g/cm3 |
|---|---|
| Boiling Point | 409ºC at 760 mmHg |
| Molecular Formula | C21H25NO |
| Molecular Weight | 307.42900 |
| Flash Point | 120.1ºC |
| Exact Mass | 307.19400 |
| PSA | 12.47000 |
| LogP | 4.35570 |
| Index of Refraction | 1.604 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
86-13-5 |
| Literature: Pedersen, Hanne; Sinning, Steffen; Buelow, Anne; Wiborg, Ove; Falborg, Lise; Bols, Mikael Organic and Biomolecular Chemistry, 2004 , vol. 2, # 19 p. 2861 - 2869 |
|
~% 
86-13-5 |
| Literature: Pedersen, Hanne; Sinning, Steffen; Buelow, Anne; Wiborg, Ove; Falborg, Lise; Bols, Mikael Organic and Biomolecular Chemistry, 2004 , vol. 2, # 19 p. 2861 - 2869 |
|
~% 
86-13-5 |
| Literature: Pedersen, Hanne; Sinning, Steffen; Buelow, Anne; Wiborg, Ove; Falborg, Lise; Bols, Mikael Organic and Biomolecular Chemistry, 2004 , vol. 2, # 19 p. 2861 - 2869 |
|
~% 
86-13-5 |
| Literature: Pedersen, Hanne; Sinning, Steffen; Buelow, Anne; Wiborg, Ove; Falborg, Lise; Bols, Mikael Organic and Biomolecular Chemistry, 2004 , vol. 2, # 19 p. 2861 - 2869 |
|
~% 
86-13-5 |
| Literature: Pedersen, Hanne; Sinning, Steffen; Buelow, Anne; Wiborg, Ove; Falborg, Lise; Bols, Mikael Organic and Biomolecular Chemistry, 2004 , vol. 2, # 19 p. 2861 - 2869 |
|
~% 
86-13-5 |
| Literature: Pedersen, Hanne; Sinning, Steffen; Buelow, Anne; Wiborg, Ove; Falborg, Lise; Bols, Mikael Organic and Biomolecular Chemistry, 2004 , vol. 2, # 19 p. 2861 - 2869 |
|
~% 
86-13-5 |
| Literature: Merck and Co.Inc. Patent: US2706198 , 1952 ; |
|
~% 
86-13-5 |
| Literature: Merck and Co.Inc. Patent: US2595405 , 1949 ; |
|
~82% 
86-13-5 |
| Literature: Pedersen, Hanne; Sinning, Steffen; Buelow, Anne; Wiborg, Ove; Falborg, Lise; Bols, Mikael Organic and Biomolecular Chemistry, 2004 , vol. 2, # 19 p. 2861 - 2869 |