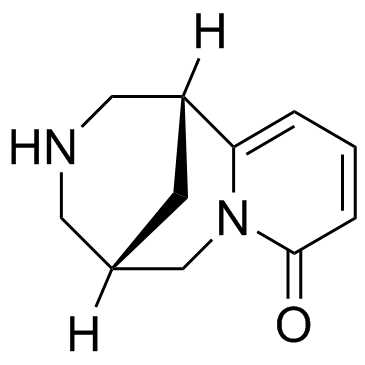
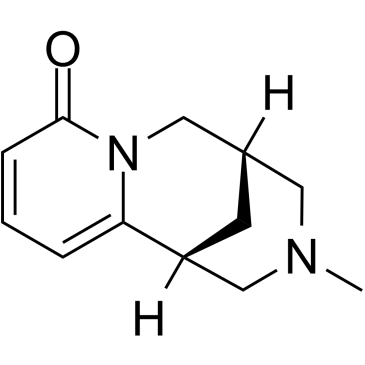
485-35-8
| Name | cytisine |
|---|---|
| Synonyms |
CYTISINE
(1R,9S)-7,11-Diazatricyclo[7.3.1.0]trideca-2,4-dien-6-one Cytitone (1R)-1,2,3,4,5,6-Hexahydro-1,5-methano-8H-pyrido-[1,2-a][1,5]diazocin-8-one (1R,5S)-3,4,5,6-Tetrahydro-1H-1,5-methanopyrido[1,2-a][1,5]diazocin-8(2H)-one MFCD00136048 Sophorin Sophorin (VAN) Ulexin Cytisine (-) Tabex Tsitafat;Lupinidine EINECS 207-616-0 Baptitoxin (1R,5S)-1,2,3,4,5,6-hexahydro-8H-1,5-methanopyrido[1,2-a][1,5]diazocin-8-one Tsitafat (-)-Cytisine Ulexine Cytiton Sophorine Cytisin Baptitoxine Laburnin |
| Description | Cytisine is an alkaloid that occurs naturally in several plant genera, such as Laburnum and Cytisus. Cytisine is a partial agonist of α4β2 nAChRs[1], and partial to full agonist at β4 containing receptors and α7 receptors[2]. has been used medically to help with smoking cessation[3]. |
|---|---|
| Related Catalog | |
| Target |
α4β2 nAChRs[1]. |
| In Vitro | Cytisine (2.5, 5 and 10 mM) is capable of inducing apoptosis in HepG2 cells[4]. Treatment with Cytisine increases the percentage of cells in the sub-G1 phase (P<0.01). The preincubation of HepG2 cells with Cytisine (2.5, 5 and 10 mM) significantly increases the sub-G1 cell population[4]. |
| In Vivo | Cytisine (5 mg/kg, i.p.) eat less and gain less weight than those that receive the vehicle[2]. Total pellet intake increases during Cytisine substitution relative to nicotine and animals self-administered Cytisine significantly less than nicotine[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 413.0±34.0 °C at 760 mmHg |
| Melting Point | 154-156ºC |
| Molecular Formula | C11H14N2O |
| Molecular Weight | 190.242 |
| Flash Point | 203.6±25.7 °C |
| Exact Mass | 190.110611 |
| PSA | 34.03000 |
| LogP | 0.07 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.623 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25;R36/37/38 |
| Safety Phrases | S26-S28-S36/37-S45 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | HA4025000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |