phenalenone
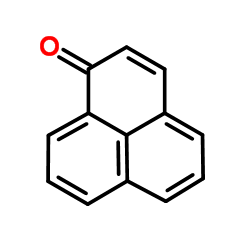
phenalenone structure
|
Common Name | phenalenone | ||
|---|---|---|---|---|
| CAS Number | 548-39-0 | Molecular Weight | 180.202 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 355.2±35.0 °C at 760 mmHg | |
| Molecular Formula | C13H8O | Melting Point | 153-156ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 157.9±20.9 °C | |
|
Enhanced Indirect Photochemical Transformation of Histidine and Histamine through Association with Chromophoric Dissolved Organic Matter.
Environ. Sci. Technol. 49 , 5511-9, (2015) Photochemical transformations greatly affect the stability and fate of amino acids (AAs) in sunlit aquatic ecosystems. Whereas the direct phototransformation of dissolved AAs is well investigated, their indirect photolysis in the presence of chromophoric diss... |
|
|
Fatty acids and vitamins generate singlet oxygen under UVB irradiation.
Exp. Dermatol. 21(2) , 135-9, (2012) UVB radiation is already known as initiator and promoter of carcinogenesis in skin. UVB is well absorbed in proteins and DNA leading to products such as cyclobutane pyrimidine dimers. In contrast, UVA radiation generates reactive oxygen species such as single... |
|
|
[Mechanism of photosensitized luminescence of singlet oxygen dimols in air-saturated pigment solutions].
Biofizika 55(3) , 389-93, (2010) Luminescence of singlet oxygen dimols (1O2)2 with the main spectral maximum at 703-706 nm and much weaker bands at 640 and 770-780 nm was studied using mechanical phosphoroscopes in aerobic solutions of the nonfluorescent photosensitizer phenalenone in CCl4 a... |
|
|
A comparative kinetic study on the singlet molecular oxygen-mediated photoxidation of alpha- and beta-chymotrypsins.
J. Pept. Res. 62(1) , 11-8, (2003) Kinetic aspects of the sensitized photooxidation of alpha- and beta-chymotrypsins have been studied at pH 6 and 8. The sensitization, employing classical O2(1Deltag)-photogenerators, such as xanthene dyes, is a kinetically intricate process because of the pre... |
|
|
Mechanism of the photochemical process of singlet oxygen production by phenalenone.
Phys. Chem. Chem. Phys. 13(9) , 4138-48, (2011) Phenalenone (PN) is a very efficient singlet oxygen sensitiser in a wide range of solvents. This work uses ab initio quantum chemical calculations (CASSCF/CASPT2 protocol) to study the mechanism for populating the triplet state of PN responsible for this reac... |
|
|
Organ-specific analysis of phenylphenalenone-related compounds in Xiphidium caeruleum.
Phytochemistry 61(7) , 819-25, (2002) The distribution pattern of phenylphenalenone-type compounds was investigated in vegetative and reproductive organs of Xiphidium caeruleum. The highest total molar concentration, up to 30 micromol g(-1) fr. wt, was detected in the root tip and the stamen. Acc... |
|
|
Oxidative biosynthesis of phenylbenzoisochromenones from phenylphenalenones.
Phytochemistry 62(3) , 307-12, (2003) 13C NMR analysis demonstrated incorporation of two 13C labelled phenylalanine units into phenylphenalenones and phenylbenzoisochromenones co-occurring in Wachendorfia thyrsiflora. These results suggest oxidative formation of phenylbenzoisochromenones followin... |
|
|
Synthesis and in vitro antiprotozoal evaluation of substituted phenalenone analogues.
Bioorg. Med. Chem. 18(12) , 4530-4, (2010) A set of derivatives encompassing structural modifications on the privileged phenalenone scaffold were assessed for their antiparasitic activities against the most clinically relevant forms of trypanosomiasis and leishmaniasis. Several compounds exhibited lei... |
|
|
Fungal phenalenones inhibit HIV-1 integrase.
J. Antibiot. 58(1) , 65-8, (2005) A phenalenone compound, atrovenetinone methyl acetal, was isolated from a culture broth of Penicillium sp. FKI-1463 as an HIV-1 integrase inhibitor, and it showed anti-HIV activity in vitro. HIV-1 integrase inhibition and anti-HIV activity of two other natura... |
|
|
Sensitized photooxidation of thyroidal hormones. Evidence for heavy atom effect on singlet molecular oxygen [O2(1Deltag)]-mediated photoreactions.
Photochem. Photobiol. 81(2) , 325-32, (2005) Thyronine derivatives are essential indicators of thyroid gland diseases in clinical diagnosis and are currently used as standards for developing ordinary biochemical assays. Photooxidation of gland hormones of the thyronine (TN) family and structurally relat... |