Fungal phenalenones inhibit HIV-1 integrase.
Kazuro Shiomi, Ryosuke Matsui, Miki Isozaki, Harumi Chiba, Takahiro Sugai, Yuichi Yamaguchi, Rokuro Masuma, Hiroshi Tomoda, Tomoko Chiba, Hua Yan, Yoshihiro Kitamura, Wataru Sugiura, Satoshi Omura, Haruo Tanaka
Index: J. Antibiot. 58(1) , 65-8, (2005)
Full Text: HTML
Abstract
A phenalenone compound, atrovenetinone methyl acetal, was isolated from a culture broth of Penicillium sp. FKI-1463 as an HIV-1 integrase inhibitor, and it showed anti-HIV activity in vitro. HIV-1 integrase inhibition and anti-HIV activity of two other natural phenalenones were also studied. Among the tested compounds, funalenone inhibited HIV-1 integrase with an IC50 value of 10 microM and showed the best selectivity (anti-HIV, IC50=1.7 microM; cytotoxicity, IC50=87 microM).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
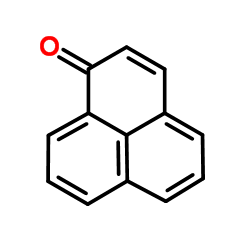 |
phenalenone
CAS:548-39-0 |
C13H8O |
|
Enhanced Indirect Photochemical Transformation of Histidine ...
2015-05-05 [Environ. Sci. Technol. 49 , 5511-9, (2015)] |
|
Fatty acids and vitamins generate singlet oxygen under UVB i...
2012-02-01 [Exp. Dermatol. 21(2) , 135-9, (2012)] |
|
[Mechanism of photosensitized luminescence of singlet oxygen...
2010-01-01 [Biofizika 55(3) , 389-93, (2010)] |
|
A comparative kinetic study on the singlet molecular oxygen-...
2003-07-01 [J. Pept. Res. 62(1) , 11-8, (2003)] |
|
Mechanism of the photochemical process of singlet oxygen pro...
2011-03-07 [Phys. Chem. Chem. Phys. 13(9) , 4138-48, (2011)] |