Synthesis and in vitro antiprotozoal evaluation of substituted phenalenone analogues.
Laura I Rosquete, M Gabriela Cabrera-Serra, José E Piñero, Patricia Martín-Rodríguez, Leandro Fernández-Pérez, Javier G Luis, Grant McNaughton-Smith, Teresa Abad-Grillo
Index: Bioorg. Med. Chem. 18(12) , 4530-4, (2010)
Full Text: HTML
Abstract
A set of derivatives encompassing structural modifications on the privileged phenalenone scaffold were assessed for their antiparasitic activities against the most clinically relevant forms of trypanosomiasis and leishmaniasis. Several compounds exhibited leishmanicidal effects at levels comparable or better than the reference drug pentamidine, while the parent phenalenone was shown to have a level of activity against Trypanosoma cruzi comparable to the marketed drug benznidazole.Copyright 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
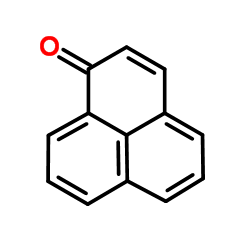 |
phenalenone
CAS:548-39-0 |
C13H8O |
|
Enhanced Indirect Photochemical Transformation of Histidine ...
2015-05-05 [Environ. Sci. Technol. 49 , 5511-9, (2015)] |
|
Fatty acids and vitamins generate singlet oxygen under UVB i...
2012-02-01 [Exp. Dermatol. 21(2) , 135-9, (2012)] |
|
[Mechanism of photosensitized luminescence of singlet oxygen...
2010-01-01 [Biofizika 55(3) , 389-93, (2010)] |
|
A comparative kinetic study on the singlet molecular oxygen-...
2003-07-01 [J. Pept. Res. 62(1) , 11-8, (2003)] |
|
Mechanism of the photochemical process of singlet oxygen pro...
2011-03-07 [Phys. Chem. Chem. Phys. 13(9) , 4138-48, (2011)] |