Phytochemistry
2003-02-01
Oxidative biosynthesis of phenylbenzoisochromenones from phenylphenalenones.
Stefan Opitz, Bernd Schneider
Index: Phytochemistry 62(3) , 307-12, (2003)
Full Text: HTML
Abstract
13C NMR analysis demonstrated incorporation of two 13C labelled phenylalanine units into phenylphenalenones and phenylbenzoisochromenones co-occurring in Wachendorfia thyrsiflora. These results suggest oxidative formation of phenylbenzoisochromenones following a late branching from a common phenylphenalenone biosynthetic pathway. A dioxygenase-type mechanism, followed by decarboxylation, is suggested for the key steps of this conversion.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
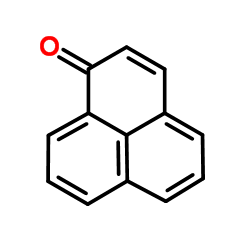 |
phenalenone
CAS:548-39-0 |
C13H8O |
Related Articles:
More...
|
Enhanced Indirect Photochemical Transformation of Histidine ...
2015-05-05 [Environ. Sci. Technol. 49 , 5511-9, (2015)] |
|
Fatty acids and vitamins generate singlet oxygen under UVB i...
2012-02-01 [Exp. Dermatol. 21(2) , 135-9, (2012)] |
|
[Mechanism of photosensitized luminescence of singlet oxygen...
2010-01-01 [Biofizika 55(3) , 389-93, (2010)] |
|
A comparative kinetic study on the singlet molecular oxygen-...
2003-07-01 [J. Pept. Res. 62(1) , 11-8, (2003)] |
|
Mechanism of the photochemical process of singlet oxygen pro...
2011-03-07 [Phys. Chem. Chem. Phys. 13(9) , 4138-48, (2011)] |