Zinc trifluoromethanesulfonate
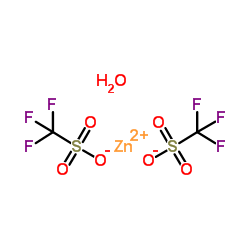
Zinc trifluoromethanesulfonate structure
|
Common Name | Zinc trifluoromethanesulfonate | ||
|---|---|---|---|---|
| CAS Number | 54010-75-2 | Molecular Weight | 381.563 | |
| Density | 4.43 | Boiling Point | 162ºC at 760 mmHg | |
| Molecular Formula | C2F6O6S2Zn | Melting Point | ≥300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
In(III)/PhCO2H binary acid catalyzed tandem [2 + 2] cycloaddition and Nazarov reaction between alkynes and acetals.
Org. Lett. 15(17) , 4496-9, (2013) A facile tandem [2 + 2] cycloaddition and Nazarov reaction has been developed. The combination of In(OTf)3 and benzoic acid was found to synergistically promote the coupling of alkynes and acetals to form 2,3-disubstituted indanones in excellent yield and dia... |
|
|
The synthesis of 3,5,6,7-tetrasubstituted isoxazolo[4,5-b]pyridines and an evaluation of their in vitro antiproliferative activity.
Adv. Clin. Exp. Med. 21(5) , 563-71, (2012) Derivatives of isoxazolopyridines exhibit diverse biological activity. One method of synthesizing isoxazolo[4,5-b]pyridines is Friedlander condensation.To establish the conditions necessary for conventional and microwave synthesis of new 3,5,6,7-tetrasubstitu... |
|
|
Michael addition/pericyclization/rearrangement--a multicomponent strategy for the synthesis of substituted resorcinols.
Org. Biomol. Chem. 10(31) , 6388-94, (2012) The combination of methyl 3,7-dioxo-2-diazo-4-octenoate from the zinc triflate catalyzed Mukaiyama-Michael reaction of methyl 3-tert-butylsilyloxy-2-diazobutenoate and 4-methoxy-3-buten-2-one with Michael acceptors (methyl vinyl ketone, N-phenylmaleimide, β-n... |
|
|
One-pot protocol to functionalized benzopyrrolizidine catalyzed successively by Rh2(OAc)4 and Cu(OTf)2: a transition metal-Lewis acid catalysis relay.
Org. Lett. 17(1) , 66-9, (2015) 4-N-allylarylpropylamino-1-sulfonyl triazoles are converted to structurally unique benzopyrrolizidinyl sulfonamides in a one-pot operation. Intramolecular capture of rhodium carbene with arylamino nitrogen gives rise to the formation of an ammonium ylide imme... |
|
|
Chemoselective synthesis of substituted pyrazoles through AgOTf-catalyzed cascade propargylic substitution-cyclization-aromatization.
Org. Biomol. Chem. 11(2) , 294-8, (2013) A cascade AgOTf-catalyzed chemoselective approach to 3,5/1,3-disubstitued pyrazoles from propargylic alcohols and para-tolylsulfonohydrazide has been developed. Good chemoselectivity is observed depending on the different substituents in the alkyne moiety of ... |
|
|
Total synthesis of (-)-cinatrin C1 based on an In(OTf)3-catalyzed Conia-Ene reaction.
J. Org. Chem. 78(8) , 3847-57, (2013) The stereocontrolled total synthesis of (-)-cinatrin C1, a phospholipase A2 inhibitor, has been accomplished. The key feature includes the stereoselective construction of the highly substituted tetrahydrofuran core by In(OTf)3-catalyzed Conia-ene reaction of ... |
|
|
In situ formation of β-glycosyl imidinium triflate from participating thioglycosyl donors: elaboration to disarmed-armed iterative glycosylation.
Chem. Commun. (Camb.) 48(88) , 10910-2, (2012) β-Glycosyl imidinium triflate is generated from participating thioglycoside donors for disarmed-armed iterative glycosylations and one-pot oligosaccharide synthesis. |
|
|
Silver triflate-catalyzed tandem reaction of N'-(2-alkynylbenzylidene)hydrazide with pyridyne.
Org. Biomol. Chem. 10(40) , 8102-7, (2012) A silver triflate-catalyzed tandem reaction of N'-(2-alkynylbenzylidene)hydrazide with pyridyne is presented. Different outcomes are obtained, depending on the pyridynes utilized in the transformation. |
|
|
An unexpected silver triflate-catalyzed tandem reaction of N'-(2-alkynylbenzylidene)hydrazide with ketene.
Chem. Commun. (Camb.) 48(56) , 7049-51, (2012) An unexpected silver triflate-catalyzed tandem reaction of N'-(2-alkynylbenzylidene)hydrazide with ketene through 6-endo-cyclization, [3+2] cycloaddition and rearrangement is reported. This reaction proceeds efficiently to generate the molecular complexity wi... |
|
|
An efficient and recyclable catalyst for the cleavage oftert-butyldiphenylsilyl ethers
Carbohydr. Res. 354 , 6-20, (2012) An efficient, chemoselective, and environment-friendly method for the deprotection of tert-butyldiphenylsilyl ethers mediated by triflic acid supported on silica gel is reported. A wide range of tert-butyldiphenylsilyl ethers derived from carbohydrate and sap... |