| Structure | Name/CAS No. | Articles |
|---|---|---|
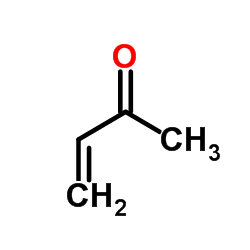 |
Methyl vinyl ketone
CAS:78-94-4 |
|
 |
lithium triflate
CAS:33454-82-9 |
|
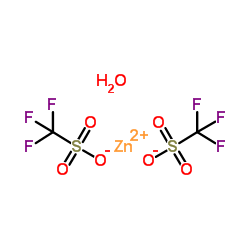 |
Zinc trifluoromethanesulfonate
CAS:54010-75-2 |
|
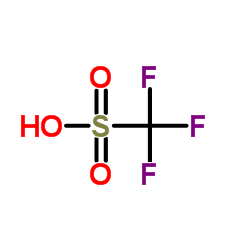 |
Trifluoromethanesulfonic acid
CAS:1493-13-6 |
|
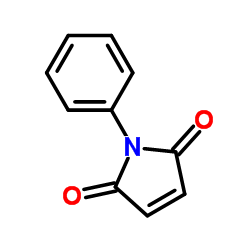 |
N-Phenylmaleimide
CAS:941-69-5 |
|
 |
Barium bis(trifluoromethanesulfonate)
CAS:2794-60-7 |