Molecules
2012-01-01
Enantioselective Michael addition of 3-aryl-substituted oxindoles to methyl vinyl ketone catalyzed by a binaphthyl-modified bifunctional organocatalyst.
Hyun Joo Lee, Saet Byeol Woo, Dae Young Kim
Index: Molecules 17(6) , 7523-32, (2012)
Full Text: HTML
Abstract
The enantioselective conjugate addition reaction of 3-aryl-substituted oxindoles with methyl vinyl ketone promoted by binaphthyl-modified bifunctional organocatalysts was investigated. The corresponding Michael adducts, containing a quaternary center at the C3-position of the oxindoles, were generally obtained in high yields with excellent enantioselectivities (up to 91% ee).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
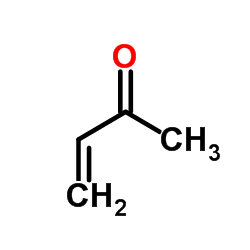 |
Methyl vinyl ketone
CAS:78-94-4 |
C4H6O |
Related Articles:
More...
|
Modulation of mitochondrial glutathione status and cellular ...
2013-05-01 [Biochem. Pharmacol. 85(9) , 1379-88, (2013)] |
|
Methyl vinyl ketone, a toxic ingredient in cigarette smoke e...
2014-01-01 [Chem. Pharm. Bull. 62(8) , 772-8, (2014)] |
|
Quantum mechanical/molecular mechanical modeling finds Diels...
2010-03-10 [J. Am. Chem. Soc. 132(9) , 3097-104, (2010)] |
|
Diabetes increases susceptibility of primary cultures of rat...
2009-11-15 [Toxicol. Appl. Pharmacol. 241(1) , 1-13, (2009)] |
|
Vinyl ketone reduction by three distinct Gluconobacter oxyda...
2008-10-01 [Appl. Microbiol. Biotechnol. 80(6) , 995-1006, (2008)] |