4-Hydroxybenzamide
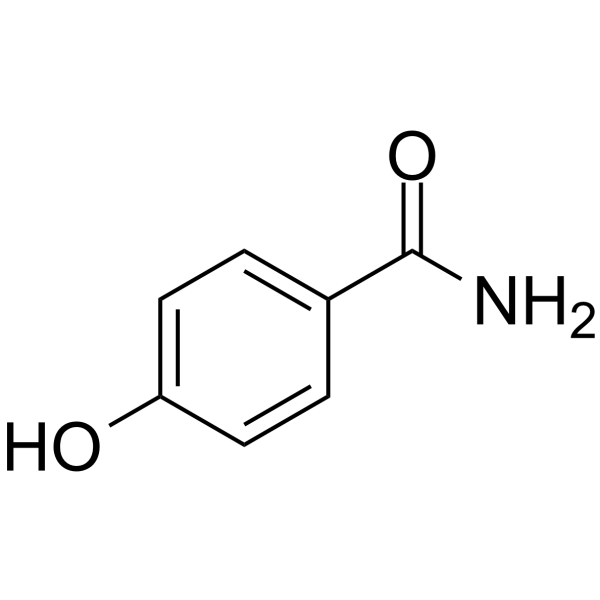
4-Hydroxybenzamide structure
|
Common Name | 4-Hydroxybenzamide | ||
|---|---|---|---|---|
| CAS Number | 619-57-8 | Molecular Weight | 137.136 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 345.3±25.0 °C at 760 mmHg | |
| Molecular Formula | C7H7NO2 | Melting Point | 161-162 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 162.6±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Influence of Secondary Interactions on the Structure, Sublimation Thermodynamics, and Solubility of Salicylate:4-Hydroxybenzamide Cocrystals. Combined Experimental and Theoretical Study.
J. Phys. Chem. B 119 , 10466-77, (2015) Cocrystal screening of 4-hydroxybenzamide with a number of salicylates (salicylic acid, SA; 4-aminosalicylic acid, PASA; acetylsalicylic acid, ASA; and salicylsalicylic acid, SSA) was conducted to confirm the formation of two cocrystals, [SA+4-OHBZA] (1:1) an... |
|
|
Synthesis and protein kinase C inhibitory activities of balanol analogs with replacement of the perhydroazepine moiety.
J. Med. Chem. 40(2) , 226-35, (1997) Balanol is a potent protein kinase C (PKC) inhibitor that is structurally composed of a benzophenone diacid, a 4-hydroxybenzamide, and a perhydroazepine ring. A number of balanol analogs in which the perhydroazepine moiety is replaced have been synthesized an... |
|
|
Energetics of the O-H bond and of intramolecular hydrogen bonding in HOC6H4C(O)Y (Y = H, CH3, CH2CH=CH2, C[triple bond]CH, CH2F, NH2, NHCH3, NO2, OH, OCH3, OCN, CN, F, Cl, SH, and SCH3) compounds.
J. Phys. Chem. A 112(40) , 10029-39, (2008) The energetics of the phenolic O-H bond in a series of 2- and 4-HOC 6H 4C(O)Y (Y = H, CH3, CH 2CH=CH2, C[triple bond]CH, CH2F, NH2, NHCH 3, NO2, OH, OCH3, OCN, CN, F, Cl, SH, and SCH3) compounds and of the intramolecular O...H hydrogen bond in 2-HOC 6H 4C(O)Y... |
|
|
Cocrystal screening of hydroxybenzamides with benzoic acid derivatives: a comparative study of thermal and solution-based methods.
Eur. J. Pharm. Sci. 65 , 56-64, (2014) The main problem occurring at the early stages of cocrystal search is the choice of an effective screening technique. Among the most popular techniques of obtaining cocrystals are crystallization from solution, crystallization from melt and solvent-drop grind... |
|
|
A novel complex of a phenolic derivative with insulin: structural features related to the T-->R transition.
Protein Sci. 5(8) , 1502-11, (1996) The structure of a symmetric T3R3f insulin hexamer, complexed with 4-hydroxybenzamide, has been determined using X-ray crystallographic techniques. Data were measured from six crystals grown in microgravity to a resolution of 1.4 A and the structure has been ... |
|
|
Structure-based design of achiral, nonpeptidic hydroxybenzamide as a novel P2/P2' replacement for the symmetry-based HIV protease inhibitors.
Bioorg. Med. Chem. 4(9) , 1471-80, (1996) A combination of structure-activity studies, kinetic analysis, X-ray crystallographic analysis, and modeling were employed in the design of a novel series of HIV-1 protease (HIV PR) inhibitors. The crystal structure of a complex of HIV PR with SRSS-2,5-bis[N-... |
|
|
Nonpeptide glycoprotein IIB/IIIA inhibitors. 19. A new design paradigm employing linearly minimized, centrally constrained, exosite inhibitors.
Bioorg. Med. Chem. Lett. 9(6) , 863-8, (1999) A new series of potent, linearly-minimized, orally active, selective GPIIb/IIIa inhibitors is identified. Thus 15 (L-750,034) achieves interaction via a constrained, non-turned conformation that maintains the proper distance between its charged termini and fu... |
|
|
Metabolism of CJ-036878, N-(3-phenethoxybenzyl)-4-hydroxybenzamide, in liver microsomes and recombinant cytochrome P450 enzymes: metabolite identification by LC-UV/MS(n) and (1)H-NMR.
Xenobiotica 37(12) , 1394-407, (2007) The identification of metabolites in the early stages of drug discovery is important not only for guiding structure-activity relationships (SAR) and structure-metabolism relationships (SMR) strategies, but also for predicting the potential for adverse events.... |
|
|
CYP3A4 and CYP3A5 catalyse the conversion of the N-methyl-D-aspartate (NMDA) antagonist CJ-036878 to two novel dimers.
Xenobiotica 37(12) , 1408-20, (2007) CJ-036878, N-(3-phenethoxybenzyl)-4-hydroxybenzamide, was developed as an antagonist of the N-methyl-D-aspartate receptor NR2B subunit. Two dimeric metabolites, CJ-047710 and CJ-047713, were identified from the incubation mixture with CJ-036878 in human liver... |