Bioorganic & Medicinal Chemistry Letters
1999-03-22
Nonpeptide glycoprotein IIB/IIIA inhibitors. 19. A new design paradigm employing linearly minimized, centrally constrained, exosite inhibitors.
G D Hartman, M E Duggan, W F Hoffman, R J Meissner, J J Perkins, A E Zartman, A M Naylor-Olsen, J J Cook, J D Glass, R J Lynch, G Zhang, R J Gould
Index: Bioorg. Med. Chem. Lett. 9(6) , 863-8, (1999)
Full Text: HTML
Abstract
A new series of potent, linearly-minimized, orally active, selective GPIIb/IIIa inhibitors is identified. Thus 15 (L-750,034) achieves interaction via a constrained, non-turned conformation that maintains the proper distance between its charged termini and full sulfonamide exosite interaction. The diminutive stature and the proposed linear conformation of L-750,034 define a new paradigm for the conceptualization of RGD mimics.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
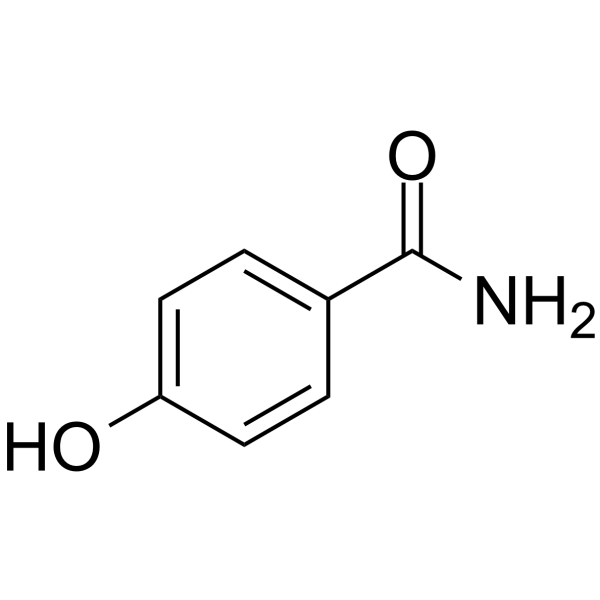 |
4-Hydroxybenzamide
CAS:619-57-8 |
C7H7NO2 |
Related Articles:
More...
|
Influence of Secondary Interactions on the Structure, Sublim...
2015-08-20 [J. Phys. Chem. B 119 , 10466-77, (2015)] |
|
Synthesis and protein kinase C inhibitory activities of bala...
1997-01-17 [J. Med. Chem. 40(2) , 226-35, (1997)] |
|
Energetics of the O-H bond and of intramolecular hydrogen bo...
2008-10-09 [J. Phys. Chem. A 112(40) , 10029-39, (2008)] |
|
Cocrystal screening of hydroxybenzamides with benzoic acid d...
2014-12-18 [Eur. J. Pharm. Sci. 65 , 56-64, (2014)] |
|
A novel complex of a phenolic derivative with insulin: struc...
1996-08-01 [Protein Sci. 5(8) , 1502-11, (1996)] |