Busulfan
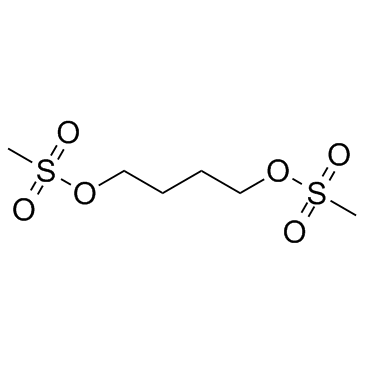
Busulfan structure
|
Common Name | Busulfan | ||
|---|---|---|---|---|
| CAS Number | 55-98-1 | Molecular Weight | 246.302 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 464.0±28.0 °C at 760 mmHg | |
| Molecular Formula | C6H14O6S2 | Melting Point | 114-117 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 234.4±24.0 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
|
Characterization of immortalized dairy goat male germline stem cells (mGSCs).
J. Cell. Biochem. 115(9) , 1549-60, (2014) Male germline stem cells (mGSCs), in charge for the fertility in male testis, are the only kind of adult stem cells that transmit genetic information to next generation, with promising prospects in germplasm resources preservation and optimization, and produc... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental drug discovery projects toward safer medicines. In this st... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI types (hepatotoxic side effects) seen in the clinic can be tra... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals can make a significant contribution to the identification o... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predictive in vivo, in vitro, and in silico models to identify comp... |
|
|
Oncostatin M maintains the hematopoietic microenvironment in the bone marrow by modulating adipogenesis and osteogenesis.
PLoS ONE 9(12) , e116209, (2015) The bone marrow (BM) is an essential organ for hematopoiesis in adult, in which proliferation and differentiation of hematopoietic stem/progenitor cells (HSPC) is orchestrated by various stromal cells. Alterations of BM hematopoietic environment lead to vario... |
|
|
Regeneration of spermatogenesis in a mouse model of azoospermia by follicle-stimulating hormone and oestradiol.
Andrologia 46(10) , 1098-106, (2014) Busulfan is a chemotherapeutic drug that induces sterility, azoospermia and testicular atrophy. To induce degeneration of spermatogenesis, we used different amounts of busulfan. Adult male C57Bl/6 mice were treated with 15, 30 and 45 mg kg(-1) of busulfan. Af... |
|
|
Hologram QSAR model for the prediction of human oral bioavailability.
Bioorg. Med. Chem. 15 , 7738-45, (2007) A drug intended for use in humans should have an ideal balance of pharmacokinetics and safety, as well as potency and selectivity. Unfavorable pharmacokinetics can negatively affect the clinical development of many otherwise promising drug candidates. A varie... |
|
|
Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors.
J. Med. Chem. 51 , 6740-51, (2008) The work provides a new model for the prediction of the MAO-A and -B inhibitor activity by the use of combined complex networks and QSAR methodologies. On the basis of the obtained model, we prepared and assayed 33 coumarin derivatives, and the theoretical pr... |
|
|
FDA-approved drug labeling for the study of drug-induced liver injury.
Drug Discov. Today 16 , 697-703, (2011) Drug-induced liver injury (DILI) is a leading cause of drugs failing during clinical trials and being withdrawn from the market. Comparative analysis of drugs based on their DILI potential is an effective approach to discover key DILI mechanisms and risk fact... |