Pleuromutilin
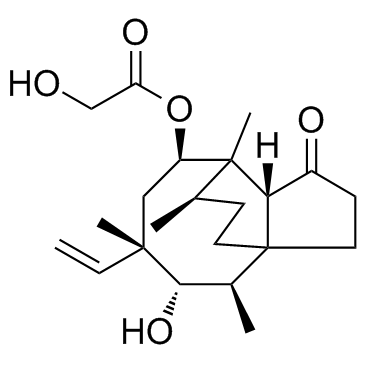
Pleuromutilin structure
|
Common Name | Pleuromutilin | ||
|---|---|---|---|---|
| CAS Number | 125-65-5 | Molecular Weight | 378.502 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 482.8±45.0 °C at 760 mmHg | |
| Molecular Formula | C22H34O5 | Melting Point | 170-171ºC | |
| MSDS | Chinese USA | Flash Point | 158.7±22.2 °C | |
|
Novel ABC transporter gene, vga(C), located on a multiresistance plasmid from a porcine methicillin-resistant Staphylococcus aureus ST398 strain.
Antimicrob. Agents Chemother. 53 , 3589-91, (2009) A novel ABC transporter gene, vga(C), was identified on the 14,365-bp multiresistance plasmid pKKS825 in a porcine methicillin (meticillin)-resistant Staphylococcus aureus isolate of sequence type 398. The vga(C) gene encodes a 523-amino-acid protein which co... |
|
|
Design, synthesis, and antibacterial activity of novel pleuromutilin derivatives bearing an amino thiazolyl ring.
Arch. Pharm. (Weinheim) 345(8) , 638-46, (2012) A series of novel pleuromutilin derivatives containing the amino thiazolyl ring were designed, synthesized, and evaluated for their antibacterial activities in vitro against Gram-positive clinical bacteria. All the target compounds showed better aqueous solub... |
|
|
Single 23S rRNA mutations at the ribosomal peptidyl transferase centre confer resistance to valnemulin and other antibiotics in Mycobacterium smegmatis by perturbation of the drug binding pocket.
Mol. Microbiol. 71(5) , 1218-27, (2009) Tiamulin and valnemulin target the peptidyl transferase centre (PTC) on the bacterial ribosome. They are used in veterinary medicine to treat infections caused by a variety of bacterial pathogens, including the intestinal spirochetes Brachyspira spp. Mutation... |
|
|
Pleuromutilin and its derivatives-the lead compounds for novel antibiotics.
Mini Rev. Med. Chem. 12(1) , 53-61, (2012) Due to the rapid onset of resistance to most antibacterial drugs, research efforts are focusing on new classes of antibacterials with different mechanisms of action from clinically used antibacterials. Pleuromutilin derivatives have received more and more sci... |
|
|
Mutilins derivatives: from veterinary to human-used antibiotics.
Mini Rev. Med. Chem. 9(12) , 1397-406, (2009) Mutilins derivatives, which were successfully developed in veterinary medicines such as tiamulin and valnemulin, have regained interest as promising antibacterial agents with potential for human use in the past few years. In 2007, Retapamulin, as the first in... |
|
|
A concise synthesis of the molecular framework of pleuromutilin.
Chem. Commun. (Camb.) 47(5) , 1500-2, (2011) Two syntheses of the tricyclic carbon skeleton of pleuromutilin are reported. Diastereoselective 1,4-conjugate additions were used to elaborate bicyclic precursors at an early stage of each route, while ring-forming olefin metatheses were executed to complete... |
|
|
Disk diffusion and MIC quality control ranges for BC-3205 and BC-3781, two novel pleuromutilin antibiotics.
J. Clin. Microbiol. 50(10) , 3361-4, (2012) MIC and disk diffusion quality control (QC) ranges were established for two new pleuromutilin antimicrobials (BC-3205 and BC-3781) in an eight-laboratory study performed according to Clinical and Laboratory Standards Institute M23-A3 guidelines. Staphylococcu... |
|
|
Novel pleuromutilin derivatives as antibacterial agents: synthesis, biological evaluation and molecular docking studies.
Bioorg. Med. Chem. Lett. 22(19) , 6166-72, (2012) Owing to the increasingly serious problems caused by multidrug resistance in community-acquired infection pathogens, it has become an urgent need to develop new classes of antibiotics for overcoming the resistance. In this paper, we describe the design and sy... |
|
|
Novel pleuromutilin derivatives with excellent antibacterial activity against Staphylococcus aureus.
Chem. Biol. Drug Des. 73(6) , 655-60, (2009) Ten novel pleuromutilin derivatives with thioether moiety and heterocyclic carboxamide or chloroformate group in the side chain were synthesized and confirmed by (1)H NMR, IR and HRMS. The results of the antibacterial activity showed that the title compounds ... |
|
|
Water-soluble phosphate prodrugs of pleuromutilin analogues with potent in vivo antibacterial activity against Gram-positive pathogens.
Bioorg. Med. Chem. Lett. 19(18) , 5407-10, (2009) A phosphate prodrug strategy was investigated to address the problem of poor aqueous solubility of pleuromutilin analogues. Water-soluble phosphate prodrugs 6a, 6b and 6c of pleuromutilin analogues were designed and synthesized. Three compounds all exhibited ... |