Bioorganic & Medicinal Chemistry Letters
2009-09-15
Water-soluble phosphate prodrugs of pleuromutilin analogues with potent in vivo antibacterial activity against Gram-positive pathogens.
Liqiang Fu, Zhiteng Jiang, Zhan Cai, Xin Liu, Huili He, Yushe Yang
Index: Bioorg. Med. Chem. Lett. 19(18) , 5407-10, (2009)
Full Text: HTML
Abstract
A phosphate prodrug strategy was investigated to address the problem of poor aqueous solubility of pleuromutilin analogues. Water-soluble phosphate prodrugs 6a, 6b and 6c of pleuromutilin analogues were designed and synthesized. Three compounds all exhibited excellent aqueous solubility (>50mg/mL) at near-neutral pH and sufficient stability in buffer solution. In particular, the phenol pleuromutilin prodrug 6c displayed favourable pharmacokinetic profiles and comparable potency with vancomycin against MSSA and MRSA strains in vivo.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
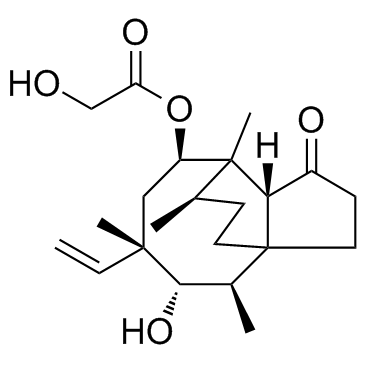 |
Pleuromutilin
CAS:125-65-5 |
C22H34O5 |
Related Articles:
More...
|
Novel ABC transporter gene, vga(C), located on a multiresist...
2009-08-01 [Antimicrob. Agents Chemother. 53 , 3589-91, (2009)] |
|
Design, synthesis, and antibacterial activity of novel pleur...
2012-08-01 [Arch. Pharm. (Weinheim) 345(8) , 638-46, (2012)] |
|
Single 23S rRNA mutations at the ribosomal peptidyl transfer...
2009-03-01 [Mol. Microbiol. 71(5) , 1218-27, (2009)] |
|
Pleuromutilin and its derivatives-the lead compounds for nov...
2012-01-01 [Mini Rev. Med. Chem. 12(1) , 53-61, (2012)] |
|
Mutilins derivatives: from veterinary to human-used antibiot...
2009-10-01 [Mini Rev. Med. Chem. 9(12) , 1397-406, (2009)] |