Safrole
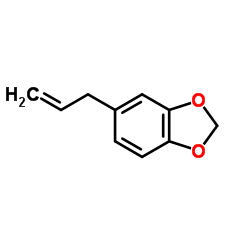
Safrole structure
|
Common Name | Safrole | ||
|---|---|---|---|---|
| CAS Number | 94-59-7 | Molecular Weight | 162.185 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 233.1±30.0 °C at 760 mmHg | |
| Molecular Formula | C10H10O2 | Melting Point | 11.2 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 97.8±0.0 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
|
Toxicological actions of plant-derived and anthropogenic methylenedioxyphenyl-substituted chemicals in mammals and insects.
J. Toxicol. Environ. Health. B. Crit. Rev. 15(6) , 365-95, (2012) The methylenedioxyphenyl (MDP) substituent is a structural feature present in many plant chemicals that deter foraging by predatory insects and herbivores. With increasing use of herbal extracts in alternative medicine, human exposure to MDP-derived plant che... |
|
|
Supported oligomethionine sulfoxide and Ellman's reagent for cysteine bridges formation.
Amino Acids 44(2) , 733-42, (2013) A large number of bioactive peptides are cyclized through a disulfide bridge. This structural feature is very important for both bioactivity and stability. The oxidation of cysteine side chains is challenging not only to avoid intermolecular reaction leading ... |
|
|
Transition-metal-free atropo-selective synthesis of biaryl compounds based on arynes.
Chemistry 18(45) , 14232-6, (2012) A modular way towards biaryls: Highly enantioenriched biphenyls can be prepared based on a transition-metal-free aryl-aryl coupling followed by efficient desymmetrization or deracemization and chemoselective functionalization (see scheme).Copyright © 2012 WIL... |
|
|
Deoxidation of fenthion sulfoxide, fenthion oxon sulfoxide and fensulfothion in gas chromatograph/mass spectrometer, and the prevention of sulfoxide deoxidation by polyethylene glycol 300.
Anal. Sci. 28(7) , 669-73, (2012) Fenthion, fenthion sulfoxide, fenthion oxon sulfoxide and fensulfothion showed two different mass spectra in GC/MS, depending on their concentrations. The base peaks shifted to lower levels by 1 m/z at lower concentration, and no retention time shifts were ob... |
|
|
L-leucine 5-hydroxylase of Nostoc punctiforme is a novel type of Fe(II)/α-ketoglutarate-dependent dioxygenase that is useful as a biocatalyst.
Appl. Microbiol. Biotechnol. 97(6) , 2467-72, (2013) L-Leucine 5-hydroxylase (LdoA) previously found in Nostoc punctiforme PCC 73102 is a novel type of Fe(II)/α-ketoglutarate-dependent dioxygenase. LdoA catalyzed regio- and stereoselective hydroxylation of L-leucine and L-norleucine into (2S,4S)-5-hydroxyleucin... |
|
|
Metabolism of 7-ethoxycoumarin, safrole, flavanone and hydroxyflavanone by cytochrome P450 2A6 variants.
Biopharm. Drug Dispos. 34(2) , 87-97, (2013) CYP 2A6 is a human enzyme that metabolizes many xenobiotics including coumarin, indole, nicotine and carcinogenic nitrosamines. The gene for CYP2A6 is polymorphic. There are few data available to clarify the relationship between P450 genetic variants and the ... |
|
|
Thiohistidine biosynthesis.
Chimia 67(5) , 333-6, (2013) Ergothioneine and ovothiol A are sulfur-containing histidine derivatives produced by microorganisms including Mycobacterium tuberculosis, Trypanosoma cruzi or Erwinia amylovora and may also play important roles in human physiology. Based on our recent identif... |
|
|
High cell density cultivation of Pseudomonas putida strain HKT554 and its application for optically active sulfoxide production.
Appl. Microbiol. Biotechnol. 97(5) , 1903-7, (2013) Culture conditions with Pseudomonas putida strain HKT554, expressing naphthalene dioxygenase, known as the biocatalyst showing wide substrate specificity, were optimized for high cell density cultivation (HCDC). Culture in a medium TK-B modified from that for... |
|
|
The metabolic activation and nucleic acid adducts of naturally-occurring carcinogens: recent results with ethyl carbamate and the spice flavors safrole and estragole.
Br. J. Cancer 48(1) , 1-15, (1983) A small (approximately 30) but varied group of organic and inorganic compounds appear to be carcinogenic in both humans and experimental animals. A much larger number and wider variety of chemical carcinogens, primarily synthetic organic compounds, are known ... |
|
|
DNA adducts derived from safrole, estragole and related compounds, and from benzene and its metabolites.
IARC Sci. Publ. (125) , 131-40, (1994)
|