| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethionine
CAS:13073-35-3 |
|
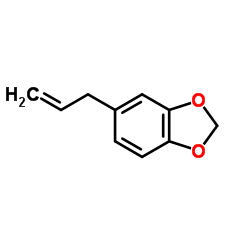 |
Safrole
CAS:94-59-7 |
|
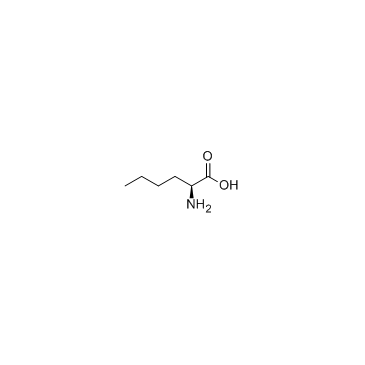 |
L-Norleucine
CAS:327-57-1 |