JohnPhos
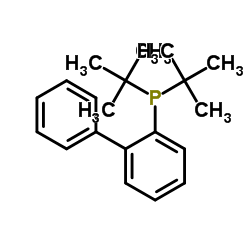
JohnPhos structure
|
Common Name | JohnPhos | ||
|---|---|---|---|---|
| CAS Number | 224311-51-7 | Molecular Weight | 298.40 | |
| Density | 1 g/cm3 | Boiling Point | 405.5±24.0 °C at 760 mmHg | |
| Molecular Formula | C20H27P | Melting Point | 86-88 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 210.1±29.2 °C | |
|
Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands.
Acc. Chem. Res. 41 , 1461, (2008) The cores of many types of polymers, ligands, natural products, and pharmaceuticals contain biaryl or substituted aromatic structures, and efficient methods of synthesizing these structures are crucial to the work of a broad spectrum of organic chemists. Rece... |
|
|
Biaryl phosphane ligands in palladium-catalyzed amination.
Angew. Chem. Int. Ed. Engl. 47 , 6338, (2008) Palladium-catalyzed amination reactions of aryl halides have undergone rapid development in the last 12 years, largely driven by the implementation of new classes of ligands. Biaryl phosphanes have proven to provide especially active catalysts in this context... |
|
|
Dialkylbiaryl Phosphines in Pd-Catalyzed Amination: A User's Guide.
Chem. Sci. 2 , 27, (2011) Dialkylbiaryl phosphines are a valuable class of ligand for Pd-catalyzed amination reactions and have been applied in a range of contexts. This review attempts to aid the reader in the selection of the best choice of reaction conditions and ligand of this cla... |
|
|
Palladium-catalyzed selective 2,3-diarylation of alpha,alpha-disubstituted 3-thiophenemethanols via cleavage of C-H and C-C bonds.
J. Org. Chem. 71 , 8309, (2006) Alpha,alpha-disubstituted 3-thiophenemethanols undergo selective diarylation accompanied by cleavage of the C-H and C-C bonds of the 2- and 3-positions, respectively, upon treatment with aryl bromides in the presence of a palladium catalyst to give the corres... |
|
|
Acid, silver, and solvent-free gold-catalyzed hydrophenoxylation of internal alkynes.
Beilstein J. Org. Chem. 9 , 2002-8, (2013) A range of arylgold compounds have been synthesized and investigated as single-component catalysts for the hydrophenoxylation of unactivated internal alkynes. Both carbene and phosphine-ligated compounds were screened as part of this work, and the most effici... |
|
|
Coordination chemistry of gold catalysts in solution: a detailed NMR study.
Chemistry 18(46) , 14732-44, (2012) Coordination chemistry of gold catalysts bearing eight different ligands [L=PPh(3), JohnPhos (L2), Xphos (L3), DTBP, IMes, IPr, dppf, S-tolBINAP (L8)] has been studied by NMR spectroscopy in solution at room temperature. Cationic or neutral mononuclear comple... |
|
|
Suzuki-Miyaura Cross-Coupling of Benzylic Bromides Under Microwave Conditions.
Tetrahedron Lett. 52(43) , 5656-5658, (2011) A procedure for benzylic Suzuki-Miyaura cross-coupling under microwave conditions has been developed. These conditions allowed for heterocyclic compounds to be coupled. Optimum conditions found were Pd(OAc)(2), JohnPhos as the catalyst and ligand, potassium c... |
|
|
Artamkina, Galina A.; et al.
Synlett 2 , 235-238, (2006)
|