Acid, silver, and solvent-free gold-catalyzed hydrophenoxylation of internal alkynes.
Marcia E Richard, Daniel V Fraccica, Kevin J Garcia, Erica J Miller, Rosa M Ciccarelli, Erin C Holahan, Victoria L Resh, Aakash Shah, Peter M Findeis, Robert A Stockland Jr
Index: Beilstein J. Org. Chem. 9 , 2002-8, (2013)
Full Text: HTML
Abstract
A range of arylgold compounds have been synthesized and investigated as single-component catalysts for the hydrophenoxylation of unactivated internal alkynes. Both carbene and phosphine-ligated compounds were screened as part of this work, and the most efficient catalysts contained either JohnPhos or IPr/SIPr. Phenols bearing either electron-withdrawing or electron-donating groups were efficiently added using these catalysts. No silver salts, acids, or solvents were needed for the catalysis, and either microwave or conventional heating afforded moderate to excellent yields of the vinyl ethers.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
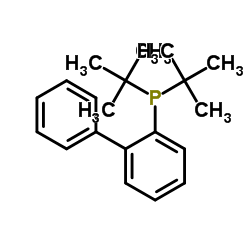 |
JohnPhos
CAS:224311-51-7 |
C20H27P |
|
Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions ...
2008-11-18 [Acc. Chem. Res. 41 , 1461, (2008)] |
|
Biaryl phosphane ligands in palladium-catalyzed amination.
2008-01-01 [Angew. Chem. Int. Ed. Engl. 47 , 6338, (2008)] |
|
Dialkylbiaryl Phosphines in Pd-Catalyzed Amination: A User's...
2012-03-01 [Chem. Sci. 2 , 27, (2011)] |
|
Palladium-catalyzed selective 2,3-diarylation of alpha,alpha...
2006-10-13 [J. Org. Chem. 71 , 8309, (2006)] |
|
Coordination chemistry of gold catalysts in solution: a deta...
2012-11-12 [Chemistry 18(46) , 14732-44, (2012)] |