4-Methylanisole
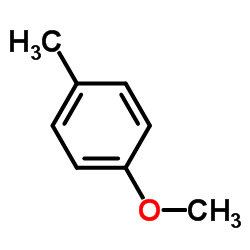
4-Methylanisole structure
|
Common Name | 4-Methylanisole | ||
|---|---|---|---|---|
| CAS Number | 104-93-8 | Molecular Weight | 122.164 | |
| Density | 0.96 | Boiling Point | 174 ºC | |
| Molecular Formula | C8H10O | Melting Point | -32°C | |
| MSDS | Chinese USA | Flash Point | 53 ºC | |
| Symbol |



GHS02, GHS07, GHS08 |
Signal Word | Warning | |
|
Identification of odorants in frankincense (Boswellia sacra Flueck.) by aroma extract dilution analysis and two-dimensional gas chromatography-mass spectrometry/olfactometry.
Phytochemistry 109 , 66-75, (2014) Frankincense has been known, traded and used throughout the ages for its exceptional aroma properties, and is still commonly used in both secular and religious settings to convey a pleasant odor. Surprisingly, the odoriferous principle(s) underlying its uniqu... |
|
|
Developmental immunotoxicity testing of 4-methyl anisole.
Regul Toxicol Pharmacol 72 , 379-85, (2015) The developmental immunotoxicity of 4-methyl anisole (4MA) was investigated in the rat. Four study designs were used, with either premating or post-weaning onset of exposure, continued to postnatal day 50, and with or without additional oral gavage of pups fr... |
|
|
Individual solvent/solute interactions through social isomerism.
J. Am. Chem. Soc. 125(46) , 13981-3, (2003) Reversible coencapsulation of a solute molecule and a single solvent molecule takes place in solution at ambient temperature. Two isomeric complexes are formed (social isomers), and their relative energies are assessed by NMR methods. Intermolecular interacti... |
|
|
An assessment of the reaction energetics for cytochrome P450-mediated reactions.
Arch. Biochem. Biophys. 385(1) , 220-30, (2001) Regioselectivity is used to determine the absolute energetic differences for four different reactions catalyzed by P450. Abstraction of a hydrogen from a benzylic carbon containing a chlorine has a 1.0 kcal/mol lower barrier than abstraction from a simple ben... |
|
|
Occurrence of aromatic methyl migration (NIH-shift) during oxidation of p-methylanisole by hemin-thiolester complex as a cytochrome P-450 model.
Biochem. Biophys. Res. Commun. 108(4) , 1649-54, (1982)
|
|
|
Four-week toxicity study of 4-methoxytoluene in rats.
Toxicol. Lett. 73(3) , 209-12, (1994) 4-Methoxytoluene was given by gavage to 4 groups of 20 rats at dosage levels of 0, 40, 120 or 240 mg kg-1 body weight/day for 4 weeks. There was a statistically significant decrease in serum creatinine and urea in the intermediate and high dose group for both... |
|
|
Vibrations and theoretical calculations of p-methylanisole in the first electronically excited S1 and ionic ground D0 states.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 67(3-4) , 824-9, (2007) One-color (1C), two-color (2C) resonant two-photon ionization (R2PI), and mass analyzed threshold ionization (MATI) methods have been applied to study the S(1)<--S(0) transition and threshold ionization of p-methylanisole. The excitation energy of the S(1)<--... |
|
|
Solubilization Site of Organic Perfume Molecules in Sodium Dodecyl Sulfate Micelles: New Insights from Proton NMR Studies.
J. Colloid. Interface Sci. 225(1) , 32-38, (2000) The site of incorporation of solubilizates in sodium dodecyl sulfate (SDS) micellar systems has been investigated by proton NMR spectroscopy. The solubilizate molecules chosen for the present study are phenol, 4-methylphenol, 4-allyl-2-methoxyphenol, anisole,... |
|
|
A concise synthesis of (+/-)-cacalol.
J. Org. Chem. 73(13) , 5177-9, (2008) A simple synthesis of the natural product cacalol has been developed that proceeds in seven steps and 21-25% overall yield. Ortho-lithiation of 4-methylanisole and alkylation with 5-iodo-1-pentene, followed by intramolecular Friedel-Crafts alkylation, gave 5-... |