| Structure | Name/CAS No. | Articles |
|---|---|---|
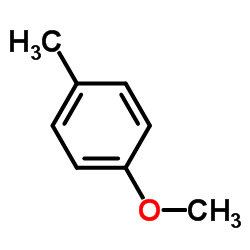 |
4-Methylanisole
CAS:104-93-8 |
Alessandro Scarso, Alexander Shivanyuk, Julius Rebek
Index: J. Am. Chem. Soc. 125(46) , 13981-3, (2003)
Full Text: HTML
Reversible coencapsulation of a solute molecule and a single solvent molecule takes place in solution at ambient temperature. Two isomeric complexes are formed (social isomers), and their relative energies are assessed by NMR methods. Intermolecular interactions between 3 aromatic solutes and 15 common solvents are evaluated.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
4-Methylanisole
CAS:104-93-8 |
C8H10O |
|
Identification of odorants in frankincense (Boswellia sacra ...
2015-01-01 [Phytochemistry 109 , 66-75, (2014)] |
|
Developmental immunotoxicity testing of 4-methyl anisole.
2015-07-01 [Regul Toxicol Pharmacol 72 , 379-85, (2015)] |
|
An assessment of the reaction energetics for cytochrome P450...
2001-01-01 [Arch. Biochem. Biophys. 385(1) , 220-30, (2001)] |
|
Occurrence of aromatic methyl migration (NIH-shift) during o...
1982-10-29 [Biochem. Biophys. Res. Commun. 108(4) , 1649-54, (1982)] |
|
Four-week toxicity study of 4-methoxytoluene in rats.
1994-09-01 [Toxicol. Lett. 73(3) , 209-12, (1994)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved