A concise synthesis of (+/-)-cacalol.
Brant L Kedrowski, Robert W Hoppe
Index: J. Org. Chem. 73(13) , 5177-9, (2008)
Full Text: HTML
Abstract
A simple synthesis of the natural product cacalol has been developed that proceeds in seven steps and 21-25% overall yield. Ortho-lithiation of 4-methylanisole and alkylation with 5-iodo-1-pentene, followed by intramolecular Friedel-Crafts alkylation, gave 5-methoxy-1,8-dimethyltetralin. This compound was then formylated in the 6-position. Baeyer-Villiger oxidation and hydrolysis of the resulting formate gave 6-hydroxy-5-methoxy-1,8-dimethyltetralin. Alkylation of the phenolic hydroxyl group with chloroacetone followed by cyclodehydration gave cacalol methyl ether. Deprotection of this aryl methyl ether yielded cacalol.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
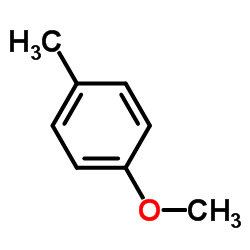 |
4-Methylanisole
CAS:104-93-8 |
C8H10O |
|
Identification of odorants in frankincense (Boswellia sacra ...
2015-01-01 [Phytochemistry 109 , 66-75, (2014)] |
|
Developmental immunotoxicity testing of 4-methyl anisole.
2015-07-01 [Regul Toxicol Pharmacol 72 , 379-85, (2015)] |
|
Individual solvent/solute interactions through social isomer...
2003-11-19 [J. Am. Chem. Soc. 125(46) , 13981-3, (2003)] |
|
An assessment of the reaction energetics for cytochrome P450...
2001-01-01 [Arch. Biochem. Biophys. 385(1) , 220-30, (2001)] |
|
Occurrence of aromatic methyl migration (NIH-shift) during o...
1982-10-29 [Biochem. Biophys. Res. Commun. 108(4) , 1649-54, (1982)] |