Isocytosine
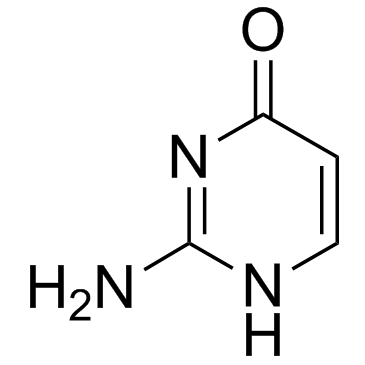
Isocytosine structure
|
Common Name | Isocytosine | ||
|---|---|---|---|---|
| CAS Number | 108-53-2 | Molecular Weight | 111.102 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 393.6±34.0 °C at 760 mmHg | |
| Molecular Formula | C4H5N3O | Melting Point | 275°C | |
| MSDS | Chinese USA | Flash Point | 191.9±25.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Lead optimization of isocytosine-derived xanthine oxidase inhibitors.
Bioorg. Med. Chem. Lett. 23(3) , 834-8, (2013) We report our attempts at improving the oral efficacy of low-nanomolar inhibitors of xanthine oxidase from isocytosine series through chemical modifications. Our lead compound had earlier shown good in vivo efficacy when administered intraperitoneally but not... |
|
|
Isocytosine-based inhibitors of xanthine oxidase: Design, synthesis, SAR, PK and in vivo efficacy in rat model of hyperuricemia
Bioorg. Med. Chem. Lett. 22(24) , 7543-6, (2012) Structure-activity relationship studies were carried out for lead generation following structure-guided design approach from an isocytosine scaffold identified earlier for xanthine oxidase inhibition. A 470-fold improvement in in vitro IC(50) was obtained in ... |
|
|
Indirect photochemical transformations of acyclovir and penciclovir in aquatic environments increase ecological risk.
Environ. Toxicol. Chem. 35 , 584-92, (2016) Acyclovir and penciclovir, 2 antiviral drugs, are increasingly detected in aquatic environments. The present study explores the natural photochemical transformation mechanisms and fate of these drugs, examining direct and indirect photochemical transformation... |
|
|
Design and synthesis of novel SATE derivatives of acyclic isocytosine and 9-deazaadenine C-nucleosides.
Nucleosides Nucleotides Nucleic Acids 29(3) , 257-66, (2010) This article describes a very simple route for synthesizing novel lipophilic phosphate bis(t-bu-SATE) prodrugs of acyclic cyclobutylated C-nucleosides such as isocytosine 12 and 9-deazaadenine 19, which were prepared from 1,1-gem cyclobutyl dicarboxylate. Syn... |
|
|
Prebiotic synthesis of diaminopyrimidine and thiocytosine.
J. Mol. Evol. 43(6) , 543-50, (1996) The reaction of guanidine hydrochloride with cyanoacetaldehyde gives high yields (40-85%) of 2,4-diaminopyrimidine under the concentrated conditions of a drying lagoon model of prebiotic synthesis, in contrast to the low yields previously obtained under more ... |
|
|
Complex formation of isocytosine tautomers with PdII and PtII.
Inorg. Chem. 43(11) , 3386-93, (2004) Isocytosine (ICH) exists in solution as two major tautomers, the keto form with N1 carrying a proton (1a) and the keto form with N3 being protonated (1b). In water, 1a and 1b exist in equilibrium with almost equal amounts of both forms present. Reactions with... |
|
|
"Paper-clip" type triple helix formation by 5'-d-(TC)3Ta(CT)3Cb(AG)3 (a and b = 0-4) as a function of loop size with and without the pseudoisocytosine base in the Hoogsteen strand.
Biochemistry 39(40) , 12457-64, (2000) The formation of a DNA "paper-clip" type triple helix (triplex) with a common sequence 5'-d-(TC)(3)T(a)()(CT)(3)C(b)()(AG)(3) (a and b = 0-4) was studied by UV thermal melting experiments and CD spectra. These DNA oligomers form triplexes and duplexes under s... |
|
|
Comparative study of the relaxation mechanisms of the excited states of cytosine and isocytosine.
J. Mol. Model. 18(12) , 5133-46, (2012) An experimental and theoretical investigation was performed to study the photostability of cytosine and isocytosine. The experimental UV irradiation of acetonitrile solutions of the two compounds showed that the amino-oxo tautomer of cytosine is photostable w... |
|
|
Beyond guanine quartets: cation-induced formation of homogenous and chimeric DNA tetraplexes incorporating iso-guanine and guanine.
Chem. Biol. 4(12) , 899-908, (1997) iso-Guanine (iso-G) is the purine component of an isomeric Watson-Crick base pair that may have existed prebiotically. By comparing the abiotic molecular recognition properties of iso-G and its complement, iso-cytosine (iso-C), with those of genomic nucleotid... |
|
|
Identification of novel isocytosine derivatives as xanthine oxidase inhibitors from a set of virtual screening hits.
Bioorg. Med. Chem. 20(9) , 2930-9, (2012) In recent years, xanthine oxidase has emerged as an important target not only for gout but also for cardiovascular and metabolic disorders involving hyperuricemia. Contrary to popular belief, recent clinical trials with uricosurics have demonstrated that enha... |