Comparative study of the relaxation mechanisms of the excited states of cytosine and isocytosine.
Rumyana I Bakalska, Vassil B Delchev
Index: J. Mol. Model. 18(12) , 5133-46, (2012)
Full Text: HTML
Abstract
An experimental and theoretical investigation was performed to study the photostability of cytosine and isocytosine. The experimental UV irradiation of acetonitrile solutions of the two compounds showed that the amino-oxo tautomer of cytosine is photostable while the amino-oxo tautomer of isocytosine tautomerizes to the amino-hydroxy form. The theoretical investigations were carried out at the CC2 level of theory. They were performed to explain the experimental observations. It was found that the (1)ππ(*) excited states of the ring deformation mechanisms of cytosine and isocytosine relax (internal conversion) to the ground states of the amino-oxo forms of the compounds. We propose a channel for the radiationless deactivation of the repulsive (1)πσ(*) excited state of the amino-oxo form of isocytosine to the ground state of the amino-hydroxy tautomer.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
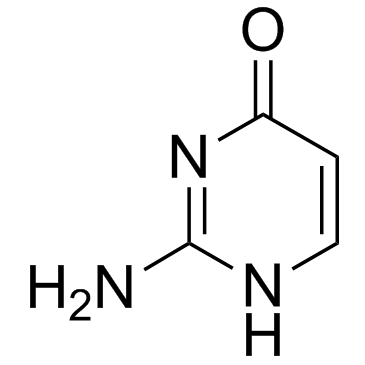 |
Isocytosine
CAS:108-53-2 |
C4H5N3O |
|
Lead optimization of isocytosine-derived xanthine oxidase in...
2013-02-01 [Bioorg. Med. Chem. Lett. 23(3) , 834-8, (2013)] |
|
Isocytosine-based inhibitors of xanthine oxidase: Design, sy...
2012-12-15 [Bioorg. Med. Chem. Lett. 22(24) , 7543-6, (2012)] |
|
Indirect photochemical transformations of acyclovir and penc...
2016-03-01 [Environ. Toxicol. Chem. 35 , 584-92, (2016)] |
|
Design and synthesis of novel SATE derivatives of acyclic is...
2010-03-01 [Nucleosides Nucleotides Nucleic Acids 29(3) , 257-66, (2010)] |
|
Prebiotic synthesis of diaminopyrimidine and thiocytosine.
1996-12-01 [J. Mol. Evol. 43(6) , 543-50, (1996)] |