Beyond guanine quartets: cation-induced formation of homogenous and chimeric DNA tetraplexes incorporating iso-guanine and guanine.
C Roberts, J C Chaput, C Switzer
Index: Chem. Biol. 4(12) , 899-908, (1997)
Full Text: HTML
Abstract
iso-Guanine (iso-G) is the purine component of an isomeric Watson-Crick base pair that may have existed prebiotically. By comparing the abiotic molecular recognition properties of iso-G and its complement, iso-cytosine (iso-C), with those of genomic nucleotide bases, it may be possible to explain the exclusion of the iso-G-iso-C base pair from modern genomes. Whether a nucleobase forms quartets may have a key role in determining its functionality. Biotically, nucleic acid tetraplexes have been implicated in cellular functions; prebiotically, tetraplexes would probably interfere with replication. Recently, in vitro selection has yielded receptors and catalysts that incorporate G quartets. The versatility of these structures could be enhanced by expanding the range of bases that can form the quartet motif.Native polyacrylamide gel electrophoresis of oligonucleotides bearing runs of iso-G provides strong support for tetraplex formation via cation-promoted DNA strand association. In particular, when strands of different lengths bearing the same iso-G tetrad recognition element were combined, five bands were observed after electrophoresis, corresponding to all possible heterotetraplexes with parallel strand alignment. An analogous experiment with a mixture of strands bearing iso-G or G tetrad recognition domains supports the existence of mixed iso-G/G tetraplexes with antiparallel strand alignment at chimeric junctions. iso-G tetraplex and quartet structure has also been probed by a photo-crosslinking experiment, ultra-violet spectroscopy and theoretical calculations.As iso-G and G both have a marked tendency to form tetraplexes, their tandem inclusion in genetic material may be problematic, leading to double-stranded DNA half composed of bases that have a tendency to auto-associate. The resulting density of 'selfish' bases could undermine Watson-Crick pair formation, especially in a prebiotic context devoid of enzymes. Nevertheless, the ability of iso-G to form mixed quartets with G may provide a basis for altering the properties of tetraplexes in the domain of artificial receptors or catalysts from in vitro selections.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
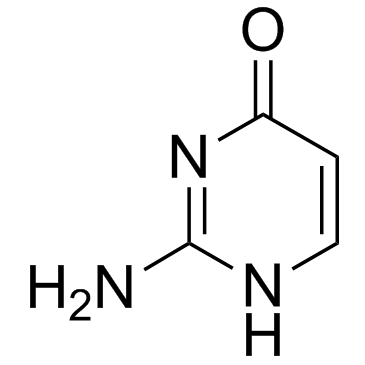 |
Isocytosine
CAS:108-53-2 |
C4H5N3O |
|
Lead optimization of isocytosine-derived xanthine oxidase in...
2013-02-01 [Bioorg. Med. Chem. Lett. 23(3) , 834-8, (2013)] |
|
Isocytosine-based inhibitors of xanthine oxidase: Design, sy...
2012-12-15 [Bioorg. Med. Chem. Lett. 22(24) , 7543-6, (2012)] |
|
Indirect photochemical transformations of acyclovir and penc...
2016-03-01 [Environ. Toxicol. Chem. 35 , 584-92, (2016)] |
|
Design and synthesis of novel SATE derivatives of acyclic is...
2010-03-01 [Nucleosides Nucleotides Nucleic Acids 29(3) , 257-66, (2010)] |
|
Prebiotic synthesis of diaminopyrimidine and thiocytosine.
1996-12-01 [J. Mol. Evol. 43(6) , 543-50, (1996)] |