Chlorosulfonylisocyanate
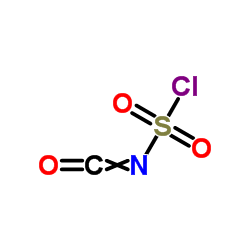
Chlorosulfonylisocyanate structure
|
Common Name | Chlorosulfonylisocyanate | ||
|---|---|---|---|---|
| CAS Number | 1189-71-5 | Molecular Weight | 141.534 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 107.0±9.0 °C at 760 mmHg | |
| Molecular Formula | CClNO3S | Melting Point | −44 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 18.5±18.7 °C | |
| Symbol |



GHS05, GHS07, GHS08 |
Signal Word | Danger | |
|
Synthesis and carbonic anhydrase inhibitory properties of sulfamides structurally related to dopamine.
Bioorg. Med. Chem. 21(11) , 2925-31, (2013) A series of novel sulfamides incorporating the dopamine scaffold were synthesized. Reaction of amines and tert-butyl-alcohol/benzyl alcohol in the presence of chlorosulfonyl isocyanate (CSI) afforded sulfamoyl carbamates, which were converted to the title com... |
|
|
Synthesis and characterization of a new class of inhibitors of membrane-associated UDP-glycosyltransferases.
J. Biol. Chem. 268(17) , 12933-8, (1993) A new class of compounds designed to inhibit membrane-associated glycosyltransferases were synthesized and their biological activities were characterized in liver microsomes and human lymphoma cell lines. These inhibitors are composed of N-acyl phenylaminoalc... |
|
|
Kinetic studies on the reaction of chlorosulfonyl isocyanate with monofluoroalkenes: experimental evidence for both stepwise and concerted mechanisms and a pre-equilibrium complex on the reaction pathway.
J. Org. Chem. 78(2) , 246-52, (2013) Chlorosulfonyl isocyanate (CSI) is reported to react with hydrocarbon alkenes by a stepwise dipolar pathway to give N-chlorosulfonyl-β-lactams that are readily reduced to β-lactams. Substitution of a vinyl hydrogen for a vinyl fluorine changes the dynamics fo... |
|
|
Synthesis of (2R,5S)-dihydroxymethyl-(3R,4R)-dihydroxypyrrolidine (DGDP) via stereoselective amination using chlorosulfonyl isocyanate.
Carbohydr. Res. 342(11) , 1502-9, (2007) A stereoselective approach for synthesizing (2R,5S)-dihydroxymethyl-(3R,4R)-dihydroxypyrrolidine 1 (2,5-dideoxy-2,5-imino-d-glucitol, DGDP) was achieved using a seven-step approach starting from 2,3,4,6-tetra-O-benzyl-d-mannose (7). Key steps for the preparat... |
|
|
Inhibitors of acyl-CoA:cholesterol O-acyltransferase. 17. Structure-activity relationships of several series of compounds derived from N-chlorosulfonyl isocyanate.
J. Med. Chem. 39(6) , 1243-52, (1996) Several series of acyl-CoA:cholesterol O-acyltransferase inhibitors were prepared by the stepwise addition of nitrogen, oxygen, and sulfur nucleophiles to N-chlorosulfonyl isocyanate. The (aminosulfonyl)ureas 3-44 were the most potent inhibitors in vitro, wit... |
|
|
Preparation of an asymmetric semipermeable membrane with anticoagulant activity.
Biomaterials 5(3) , 153-6, (1984) In this paper we report the preparation of a new asymmetric semipermeable styrene-isoprene-styrene block-copolymer membrane. Its modification by addition of gaseous N-chlorosulphonylisocyanate alters neither its permselectivity nor its water permeability rate... |
|
|
Surface modification of polycarbonate with synthetic polyelectrolyte-anticoagulant activity.
Biomater. Med. Devices. Artif. Organs 12(3-4) , 215-33, (1984) Natural rubber with C = C bonds had been modified by reaction with chlorosulfonyl isocyanate (CSI) and 70% of the products were obtained, which yielded polyelectrolyte on treatment with NaOH, having sulfamate and carboxylate groups. The polyelectrolyte showed... |
|
|
J. Org. Chem. 58 , 7022, (1993)
|
|
|
Aldrichimica Acta 10 , 23, (1977)
|
|
|
Synlett , 539, (1994)
|