Synthesis of (2R,5S)-dihydroxymethyl-(3R,4R)-dihydroxypyrrolidine (DGDP) via stereoselective amination using chlorosulfonyl isocyanate.
In Su Kim, Sin Jung Kim, Jae Koo Lee, Qing Ri Li, Young Hoon Jung
Index: Carbohydr. Res. 342(11) , 1502-9, (2007)
Full Text: HTML
Abstract
A stereoselective approach for synthesizing (2R,5S)-dihydroxymethyl-(3R,4R)-dihydroxypyrrolidine 1 (2,5-dideoxy-2,5-imino-d-glucitol, DGDP) was achieved using a seven-step approach starting from 2,3,4,6-tetra-O-benzyl-d-mannose (7). Key steps for the preparation of the title compound 1 involved the regioselective and diastereoselective amination of the cinnamyl anti-1,2-polybenzyl ethers 5 and 6 using chlorosulfonyl isocyanate (CSI) and ring cyclization to form the pyrrolidine ring. The reaction between anti-1,2-polybenzyl ether 5 and CSI in toluene at 0 degrees C afforded the corresponding anti-1,2-amino alcohol 4 as a major product with a diastereoselectivity of 16:1 in 76% yield. The mechanism underlying these reactions may be explained by the neighboring-group effect leading to the retention of stereochemistry.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
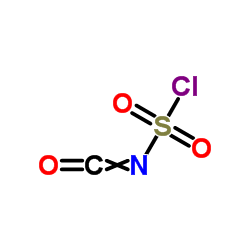 |
Chlorosulfonylisocyanate
CAS:1189-71-5 |
CClNO3S |
|
Synthesis and carbonic anhydrase inhibitory properties of su...
2013-06-01 [Bioorg. Med. Chem. 21(11) , 2925-31, (2013)] |
|
Synthesis and characterization of a new class of inhibitors ...
1993-06-15 [J. Biol. Chem. 268(17) , 12933-8, (1993)] |
|
Kinetic studies on the reaction of chlorosulfonyl isocyanate...
2013-01-18 [J. Org. Chem. 78(2) , 246-52, (2013)] |
|
Inhibitors of acyl-CoA:cholesterol O-acyltransferase. 17. St...
1996-03-15 [J. Med. Chem. 39(6) , 1243-52, (1996)] |
|
Preparation of an asymmetric semipermeable membrane with ant...
1984-05-01 [Biomaterials 5(3) , 153-6, (1984)] |