Inhibitors of acyl-CoA:cholesterol O-acyltransferase. 17. Structure-activity relationships of several series of compounds derived from N-chlorosulfonyl isocyanate.
J A Picard, P M O'Brien, D R Sliskovic, M K Anderson, R F Bousley, K L Hamelehle, B R Krause, R L Stanfield
Index: J. Med. Chem. 39(6) , 1243-52, (1996)
Full Text: HTML
Abstract
Several series of acyl-CoA:cholesterol O-acyltransferase inhibitors were prepared by the stepwise addition of nitrogen, oxygen, and sulfur nucleophiles to N-chlorosulfonyl isocyanate. The (aminosulfonyl)ureas 3-44 were the most potent inhibitors in vitro, with several compounds having IC50 values < 1 microM. Although the other series of compounds were not as potent in vitro, many compounds did display good in vivo activity in cholesterol-fed rats. Several of the oxysulfonyl carbamates (including CI-999, 115) showed excellent lipid-lowering activity in the chronic in vivo screen, demonstrating significant cholesterol lowering in a pre-established hypercholesterolemic state.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
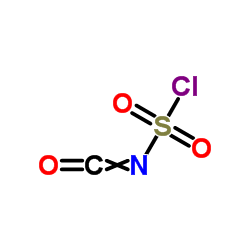 |
Chlorosulfonylisocyanate
CAS:1189-71-5 |
CClNO3S |
|
Synthesis and carbonic anhydrase inhibitory properties of su...
2013-06-01 [Bioorg. Med. Chem. 21(11) , 2925-31, (2013)] |
|
Synthesis and characterization of a new class of inhibitors ...
1993-06-15 [J. Biol. Chem. 268(17) , 12933-8, (1993)] |
|
Kinetic studies on the reaction of chlorosulfonyl isocyanate...
2013-01-18 [J. Org. Chem. 78(2) , 246-52, (2013)] |
|
Synthesis of (2R,5S)-dihydroxymethyl-(3R,4R)-dihydroxypyrrol...
2007-08-13 [Carbohydr. Res. 342(11) , 1502-9, (2007)] |
|
Preparation of an asymmetric semipermeable membrane with ant...
1984-05-01 [Biomaterials 5(3) , 153-6, (1984)] |