Azetidine
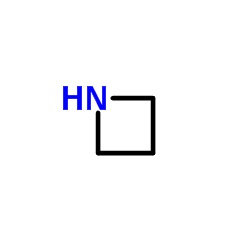
Azetidine structure
|
Common Name | Azetidine | ||
|---|---|---|---|---|
| CAS Number | 503-29-7 | Molecular Weight | 57.094 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 64.9±8.0 °C at 760 mmHg | |
| Molecular Formula | C3H7N | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | -23.6±16.5 °C | |
| Symbol |


GHS02, GHS05 |
Signal Word | Danger | |
|
Synthesis from D-altrose of (5R,6R,7R,8S)-5,7-dihydroxy-8-hydroxymethylconidine and 2,4-dideoxy-2,4-imino-D-glucitol, azetidine analogues of swainsonine and 1,4-dideoxy-1,4-imino-D-mannitol.
Org. Lett. 14(16) , 4174-7, (2012) Ring closure of a 3,5-di-O-triflate derived from D-altrose with benzylamine allowed the formation of both monocyclic and bicyclic azetidine analogues of swainsonine. |
|
|
Identification of spirocyclic piperidine-azetidine inverse agonists of the ghrelin receptor.
Bioorg. Med. Chem. Lett. 22(13) , 4281-7, (2012) The discovery of spirocyclic piperidine-azetidine inverse agonists of the ghrelin receptor is described. The characterization and redressing of the issues associated with these compounds is detailed. An efficient three-step synthesis and a binding assay were ... |
|
|
Complex N-heterocycle synthesis via iron-catalyzed, direct C-H bond amination.
Science 340(6132) , 591-5, (2013) The manipulation of traditionally unreactive functional groups is of paramount importance in modern chemical synthesis. We have developed an iron-dipyrrinato catalyst that leverages the reactivity of iron-borne metal-ligand multiple bonds to promote the direc... |
|
|
Chemistry and pharmacology of nicotinic ligands based on 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol (AMOP-H-OH) for possible use in depression.
ChemMedChem 4(8) , 1279-91, (2009) AMOP-H-OH (sazetidine-A; 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol) and some sulfur-bearing analogues were tested for their activities in vitro against human alpha4beta2-, alpha4beta4-, alpha3beta4*- and alpha1*-nicotinic acetylcholine receptors (... |
|
|
Fatty acid amide hydrolase inhibitors. Surprising selectivity of chiral azetidine ureas.
Bioorg. Med. Chem. Lett. 19(15) , 4241-4, (2009) We report the discovery of a novel, chiral azetidine urea inhibitor of Fatty Acid Amide Hydrolase (FAAH,) and describe the surprising species selectivity of VER-156084 versus rat and human FAAH and also hCB1. |
|
|
A theoretical study of dihydrogen bonds in small protonated rings: aziridine and azetidine cations.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 75(2) , 563-6, (2010) B3LYP/6-311++G(d,p) calculations were used to predict some molecular properties of the C2H6N+...BeH2, C2H6N...MgH2, C3H8N...BeH2 and C3H8N+...MgH2 dihydrogen-bonded complexes. In these systems, it was demonstrated that the C2H6N+ and C3H8N+ protonated rings a... |
|
|
Synthesis and functionalization of 3-alkylidene-1,2-diazetidines using transition metal catalysis.
Org. Lett. 13(7) , 1686-9, (2011) An efficient two-step synthesis of a wide range of 3-methylene-1,2-diazetidines has been developed through application of a Cu(I)-catalyzed 4-exo ring closure. The double bond of this new class of strained heterocycle can be functionalized in a stereocontroll... |
|
|
Azetidine-based inhibitors of dipeptidyl peptidase IV (DPP IV).
Curr. Top. Med. Chem 7(6) , 597-608, (2007) The structure-activity relationships of azetidine-based DPP IV inhibitors will be discussed in detail in the following review. The azetidine-based DPP IV inhibitors can be divided into three main subtypes, the 2-cyanoazetidines, 3-fluoroazetidines and 2-ketoa... |
|
|
Fatty acid amide hydrolase inhibitors. 3: tetra-substituted azetidine ureas with in vivo activity.
Bioorg. Med. Chem. Lett. 22(2) , 901-6, (2012) We describe here our attempts to optimise the human fatty acid amide hydrolase (FAAH) inhibition and physicochemical properties of our previously reported tetrasubstituted azetidine urea FAAH inhibitor, VER-156084. We describe the SAR of a series of analogues... |
|
|
Azetidine derivatives as novel γ-aminobutyric acid uptake inhibitors: Synthesis, biological evaluation, and structure–activity relationship
Eur. J. Med. Chem. 45 , 2453-66, (2010) In this study azetidine derivatives representing conformationally constrained GABA or β-alanine analogs were evaluated for their potency as GABA-uptake inhibitors. The study comprised derivatives substituted in 2- as well as in 3-position with either an aceti... |