Chemistry and pharmacology of nicotinic ligands based on 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol (AMOP-H-OH) for possible use in depression.
Alan P Kozikowski, J Brek Eaton, Krishna Mohan Bajjuri, Sheela K Chellappan, Yihua Chen, Sudhakar Karadi, Rong He, Barbara Caldarone, Michael Manzano, Po-Wai Yuen, Ronald J Lukas
Index: ChemMedChem 4(8) , 1279-91, (2009)
Full Text: HTML
Abstract
AMOP-H-OH (sazetidine-A; 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol) and some sulfur-bearing analogues were tested for their activities in vitro against human alpha4beta2-, alpha4beta4-, alpha3beta4*- and alpha1*-nicotinic acetylcholine receptors (nAChRs). AMOP-H-OH was also assessed in an antidepressant efficacy model. AMOP-H-OH and some of its analogues have high potency and selectivity for alpha4beta2-nAChRs over other nAChR subtypes. Effects are manifested as partial agonism, perhaps reflecting selectivity for high sensitivity (alpha4)(3)(beta2)(2)-nAChRs. More prolonged exposure to AMOP-H-OH and its analogues produces inhibition of subsequent responses to acute challenges with full nicotinic agonists, again selectively for alpha4beta2-nAChRs over other nAChR subtypes. The inhibition is mediated either via antagonism or desensitization of nAChR function, but the degree of inhibition of alpha4beta2-nAChRs is limited by the partial agonist activity of the drugs. Certain aspects of the in vitro pharmacology suggest that AMOP-H-OH and some of its analogues have a set of binding sites on alpha4beta2-nAChRs that are distinct from those for full agonists. The in vitro pharmacological profile suggests that peripheral side effects of AMOP-H-OH or its analogues would be minimal and that their behavioral effects would be dominated by central nAChR actions. AMOP-H-OH also has profound and high potency antidepressant-like effects in the forced swim test. The net action of prolonged exposure to AMOP-H-OH or its analogues, as for nicotine, seems to be a selective decrease in alpha4beta2-nAChR function. Inactivation of nAChRs may be a common neurochemical endpoint for nicotine dependence, its treatment, and some of its manifestations, including relief from depression.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
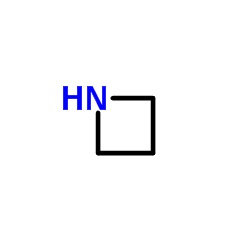 |
Azetidine
CAS:503-29-7 |
C3H7N |
|
Synthesis from D-altrose of (5R,6R,7R,8S)-5,7-dihydroxy-8-hy...
2012-08-17 [Org. Lett. 14(16) , 4174-7, (2012)] |
|
Identification of spirocyclic piperidine-azetidine inverse a...
2012-07-01 [Bioorg. Med. Chem. Lett. 22(13) , 4281-7, (2012)] |
|
Complex N-heterocycle synthesis via iron-catalyzed, direct C...
2013-05-03 [Science 340(6132) , 591-5, (2013)] |
|
Fatty acid amide hydrolase inhibitors. Surprising selectivit...
2009-08-01 [Bioorg. Med. Chem. Lett. 19(15) , 4241-4, (2009)] |
|
A theoretical study of dihydrogen bonds in small protonated ...
2010-02-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 75(2) , 563-6, (2010)] |