Azetidine-based inhibitors of dipeptidyl peptidase IV (DPP IV).
Dana Ferraris, Sergei Belyakov, Weixing Li, Eddie Oliver, Yao-Sen Ko, David Calvin, Susan Lautar, Bert Thomas, Camilo Rojas
Index: Curr. Top. Med. Chem 7(6) , 597-608, (2007)
Full Text: HTML
Abstract
The structure-activity relationships of azetidine-based DPP IV inhibitors will be discussed in detail in the following review. The azetidine-based DPP IV inhibitors can be divided into three main subtypes, the 2-cyanoazetidines, 3-fluoroazetidines and 2-ketoazetidines. These subtypes have been explored and structure-activity relationships have been established by several groups. Several compounds within each of these subtypes display sub micromolar potency against DPP IV. The most potent cyanoazetidines and ketoazetidines have large, hydrophobic amino acid groups bound to the azetidine nitrogen and display activities below 100 nM. DPP IV inhibition is not sensitive to stereochemistry at the 2-position as both 2-(R)- and 2-(S)-cyano and -keto azetidines display similar inhibitory potencies. While these "warhead"-based cyano- and ketoazetidines have the potential for covalent, bond-forming inhibition, they can also react to internally cyclize into inactive ketopiperazines and dihydroketopyrazine. Thus, chemical instability was also explored for compounds in these two subtypes and certain members of the cyanoazetidine series display aqueous stability comparable to the closely related cyanopyrrolidines. Select 3-fluoroazetidines also display inhibitory potencies below 1 microM without the propensity for cyclization and chemical instability associated with the other subseries.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
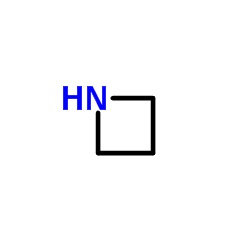 |
Azetidine
CAS:503-29-7 |
C3H7N |
|
Synthesis from D-altrose of (5R,6R,7R,8S)-5,7-dihydroxy-8-hy...
2012-08-17 [Org. Lett. 14(16) , 4174-7, (2012)] |
|
Identification of spirocyclic piperidine-azetidine inverse a...
2012-07-01 [Bioorg. Med. Chem. Lett. 22(13) , 4281-7, (2012)] |
|
Complex N-heterocycle synthesis via iron-catalyzed, direct C...
2013-05-03 [Science 340(6132) , 591-5, (2013)] |
|
Chemistry and pharmacology of nicotinic ligands based on 6-[...
2009-08-01 [ChemMedChem 4(8) , 1279-91, (2009)] |
|
Fatty acid amide hydrolase inhibitors. Surprising selectivit...
2009-08-01 [Bioorg. Med. Chem. Lett. 19(15) , 4241-4, (2009)] |