UDPGA
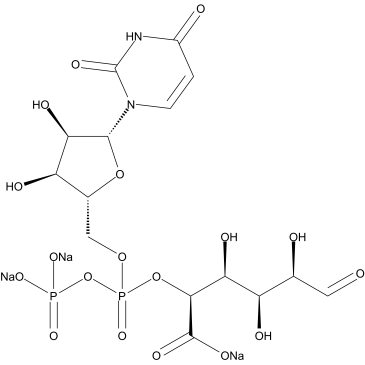
UDPGA structure
|
Common Name | UDPGA | ||
|---|---|---|---|---|
| CAS Number | 63700-19-6 | Molecular Weight | 646.231 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C15H19N2Na3O18P2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
|
In vitro and in vivo biotransformation of WMS-1410, a potent GluN2B selective NMDA receptor antagonist.
J. Pharm. Biomed. Anal. 94 , 36-44, (2014) Structural modification of the GluN2B selective NMDA receptor antagonist ifenprodil led to the 3-benzazepine WMS-1410 with similar GluN2B affinity but higher receptor selectivity. Herein the in vitro and in vivo biotransformation of WMS-1410 is reported. Incu... |
|
|
Metabolic drug-drug interaction potential of macrolactin A and 7-O-succinyl macrolactin A assessed by evaluating cytochrome P450 inhibition and induction and UDP-glucuronosyltransferase inhibition in vitro.
Antimicrob. Agents Chemother. 58(9) , 5036-46, (2014) Macrolactin A (MA) and 7-O-succinyl macrolactin A (SMA), polyene macrolides containing a 24-membered lactone ring, show antibiotic effects superior to those of teicoplanin against vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureu... |
|
|
Simultaneous determination of intracellular UDP-sugars in hyaluronic acid-producing Streptococcus zooepidemicus.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 997 , 194-9, (2015) Two chromatographic methods for the quantitative analysis of uridine diphosphate (UDP) sugars involved in hyaluronan pathway of Streptococcus zooepidemicus (SEZ) were developed and compared. The sample preparation protocol using centrifugation and extraction ... |
|
|
Cytochrome P450 3A-mediated metabolism of the topoisomerase I inhibitor 9-aminocamptothecin: impact on cancer therapy.
Int. J. Oncol. 45(2) , 877-86, (2014) The metabolism of 9-aminocamptothecin (9-AC) was investigated in human and rat liver microsomes. In both species 9-AC was almost exclusively biotransformed to dihydroxy-9-AC (M1) and monohydroxy-9-AC (M2). The enzymatic efficiencies of the formation of M1 and... |
|
|
Identification of UDP-glucuronosyltransferase isoforms responsible for leonurine glucuronidation in human liver and intestinal microsomes.
Xenobiotica 44(9) , 775-84, (2014) Leonurine is a potent component of herbal medicine Herba leonuri. The detail information on leonurine metabolism in human has not been revealed so far. Two primary metabolites, leonurine O-glucuronide and demethylated leonurine, were observed and identified i... |
|
|
Biotransformation of glucoaurantio-obtusin towards aurantio-obtusin increases the toxicity of irinotecan through increased inhibition towards SN-38 glucuronidation.
Phytother Res. 28(10) , 1577-80, (2014) The present study aims to investigate the influence of irinotecan's toxicity by the biotransformation of glucoaurantio-obtusin to aurantio-obtusin. Intraperitoneal administration (i.p.) of 100 mg/kg aurantio-obtusin significantly increased the toxicity of iri... |
|
|
Glucuronidation of aurantio-obtusin: identification of human UDP-glucuronosyltransferases and species differences.
Xenobiotica 44(8) , 716-21, (2014) 1. The aurantio-obtusin's glucuronide was detected when aurantio-obtusin was incubated with human liver microsomes (HLMs). Recombinant UGT isoforms screening experiment showed that UGT1A8 was the major isoform contributed to the glucuronidation. 2. The metabo... |