| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
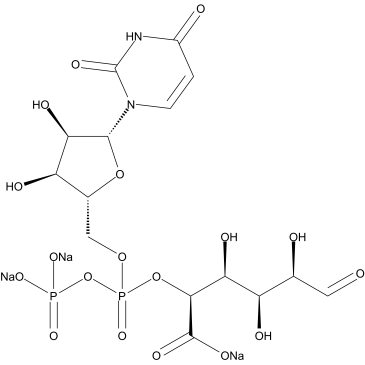 |
UDPGA
CAS:63700-19-6 |
|
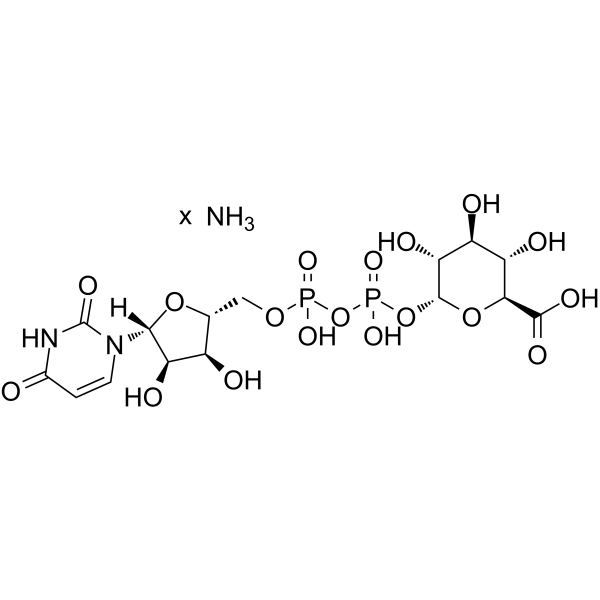 |
Uridine diphosphate glucuronic acid ammonium
CAS:43195-60-4 |
|
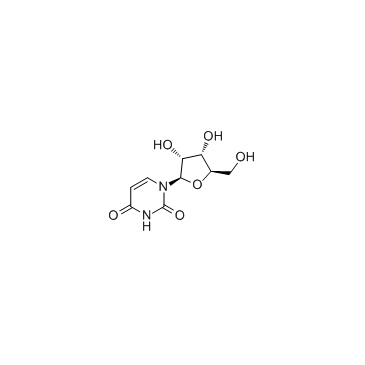 |
Uridine
CAS:58-96-8 |
|
![5'-[[(3S)-3-AMINO-3-CARBOXYPROPYL]METHYLSULFONIO]-5'-DEOXY-ADENOSINE IODIDE Structure](https://image.chemsrc.com/caspic/439/3493-13-8.png) |
5'-[[(3S)-3-AMINO-3-CARBOXYPROPYL]METHYLSULFONIO]-5'-DEOXY-ADENOSINE IODIDE
CAS:3493-13-8 |