4-Hydroxycoumarin
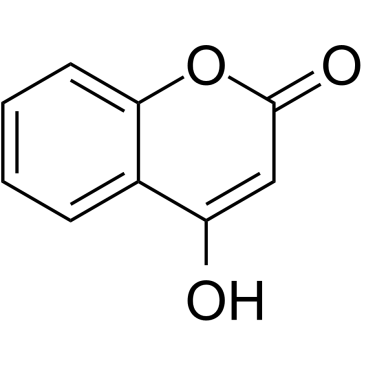
4-Hydroxycoumarin structure
|
Common Name | 4-Hydroxycoumarin | ||
|---|---|---|---|---|
| CAS Number | 1076-38-6 | Molecular Weight | 162.142 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 352.4±42.0 °C at 760 mmHg | |
| Molecular Formula | C9H6O3 | Melting Point | 211-213 °C(lit.) | |
| MSDS | USA | Flash Point | 165.4±20.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Comparative Study of 17-AAG and NVP-AUY922 in Pancreatic and Colorectal Cancer Cells: Are There Common Determinants of Sensitivity?
Transl. Oncol. 7(5) , 590-604, (2014) The use of heat shock protein 90 (Hsp90) inhibitors is an attractive antineoplastic therapy. We wanted to compare the effects of the benzoquinone 17-allylamino-17-demethoxygeldanamycin (17-AAG, tanespimycin) and the novel isoxazole resorcinol-based Hsp90 inhi... |
|
|
Potential antiproliferative effect of isoxazolo- and thiazolo coumarin derivatives on breast cancer mediated bone and lung metastases.
Acta. Pharm. 65(1) , 53-63, (2015) The study highlights the current progress in the development of coumarin scaffolds for drug discovery as novel anticancer agents in metastatic breast cancer. Eight compounds, combining the coumarin core and five membered heterocycles (isoxazoles and thiazoles... |
|
|
Design, synthesis, and evaluation of coumarin-based molecular probes for imaging of myelination.
J. Med. Chem. 54 , 2331-40, (2011) Myelination represents one of the most fundamental biological processes in the vertebrate nervous system. Abnormalities and changes in myelination in the central nervous system (CNS) are seen in many neurodegenerative disorders, such as multiple sclerosis (MS... |
|
|
6,7-Dihydroxy-4-phenylcoumarin as inhibitor of aldose reductase 2.
Bioorg. Med. Chem. Lett. 20 , 5630-3, (2010) We report the structure-activity relationship of a series of coumarins as aldose reductase 2 (ALR2) inhibitors and their suppressive effect on the accumulation of galactitol in the rat lens. We evaluated their ALR2 selectivity profile against sorbitol dehydro... |
|
|
Synthesis and free radical scavenging activity of some new spiropyranocoumarins.
Bioorg. Med. Chem. Lett. 18 , 5781-4, (2008) A series of novel spiro-substituted 4-hydroxypyranocoumarins and their corresponding dihydropyrano cis-diols has been synthesized. Among them the spiroadamantylpyranocoumarin and the diols can interact with the stable free radical 1,1-diphenyl-2-picrylhydrazy... |
|
|
Gas phase retro-Michael reaction resulting from dissociative protonation: fragmentation of protonated warfarin in mass spectrometry.
J. Mass Spectrom. 47(8) , 1059-64, (2012) A mass spectrometric study of protonated warfarin and its derivatives (compounds 1 to 5) has been performed. Losses of a substituted benzylideneacetone and a 4-hydroxycoumarin have been observed as a result of retro-Michael reaction. The added proton is initi... |
|
|
Relationship between quantum-chemical descriptors of proton dissociation and experimental acidity constants of various hydroxylated coumarins. Identification of the biologically active species for xanthine oxidase inhibition.
Eur. J. Med. Chem. 42 , 1028-31, (2007) Quantum-chemical descriptors related to proton dissociation constants of a set of coumarins hydroxylated in various positions have been computed and related to the experimental pK(a) values. An excellent correlation was found between the computed deprotonatio... |
|
|
Total synthesis of flocoumafen via knoevenagel condensation and intramolecular ring cyclization: general access to natural products.
Molecules 17(2) , 2091-102, (2012) The total synthesis and structure determination of cis- and trans-flocoumafen was described. The key synthetic steps involve Knoevenagel condensation with p-methoxybenzaldehyde, in situ decarboxylation and intramolecular ring cyclization to construct the tetr... |
|
|
Inhibition of human DHODH by 4-hydroxycoumarins, fenamic acids, and N-(alkylcarbonyl)anthranilic acids identified by structure-guided fragment selection.
ChemMedChem 5(4) , 608-17, (2010) A strategy that combines virtual screening and structure-guided selection of fragments was used to identify three unexplored classes of human DHODH inhibitor compounds: 4-hydroxycoumarins, fenamic acids, and N-(alkylcarbonyl)anthranilic acids. Structure-guide... |
|
|
Coumarins incorporating hydroxy- and chloro-moieties selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II.
Bioorg. Med. Chem. Lett. 20 , 4511-4, (2010) A series of coumarins incorporating hydroxy-, chloro- and/or chloromethyl-moieties in positions 3-, 4-, 6- and 7- of the heterocyclic ring were investigated for the inhibition of the zinc enzyme carbonic anhydrase (CA, EC 4.2.1.1). These coumarins were very w... |