Nicotinohydrazide
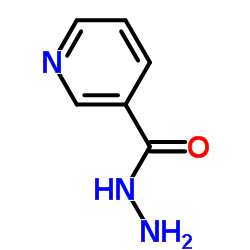
Nicotinohydrazide structure
|
Common Name | Nicotinohydrazide | ||
|---|---|---|---|---|
| CAS Number | 553-53-7 | Molecular Weight | 137.139 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C6H7N3O | Melting Point | 159-161 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Controlled release studies of antimalarial 1, 3, 5-trisubstituted-2-pyrazolines from biocompatible chitosan-heparin Layer-by-Layer (LbL) self assembled thin films.
Colloids Surf. B Biointerfaces 125 , 151-9, (2015) Herein we report the in-vitro controlled release properties of 1, 3, 5-trisubstituted-2-pyrazolines through Layer-by-Layer (LbL) self assembled thin films fabricated from chitosan and heparin sodium salt as biocompatible polyelectrolytes. This study was carri... |
|
|
Chemokine CCL17 induced by hypoxia promotes the proliferation of cervical cancer cell.
Am. J. Cancer Res. 5 , 3072-84, (2015) Cervical cancer is often associated with hypoxia and many kinds of chemokines. But the relationship and role of hypoxia and Chemokine (C-C motif) ligand 17 (CCL17) in cervical cancer are still unknown. Here, we found that CCL17 was high expressed in cervical ... |
|
|
Double-level "orthogonal" dynamic combinatorial libraries on transition metal template.
Proc. Natl. Acad. Sci. U. S. A. 98(4) , 1347-52, (2001) Dynamic combinatorial libraries are mixtures of compounds that exist in a dynamic equilibrium and can be driven to compositional self adaptation via selective binding of a specific assembly of certain components to a molecular target. We present here an exten... |
|
|
Peroxidase-mediated oxidation of isoniazid.
Antimicrob. Agents Chemother. 27(3) , 399-403, (1985) Oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase at the expense of H2O2 yielded reactive species which were able to reduce nitroblue tetrazolium and bleach p-nitrosodimethylaniline. Nicotinic acid hydrazide oxidation did not caus... |
|
|
T cell-mediated induction of thymic stromal lymphopoietin in differentiated human primary bronchial epithelial cells.
Clin. Exp. Allergy 44(7) , 953-64, (2014) Inhaled peptide challenge has been shown to induce T cell-mediated, isolated late asthmatic reaction (LAR), characterized by recruitment of CD4(+) T cells and increased levels of thymus and activation-regulated chemokine (TARC; CCL17). Epithelial-derived thym... |
|
|
[Tautomerism of 2-hydrazino-4-phenylthiazole<-->4-phenylthiazol-2-one hydrazone. Derivatives of acids. II. (4-phenyl-3-R-thiazol-2-ylidene) and beta-methyl-beta-(4-phenylthiazol-2-yl) hydrazides of picolinic, nicotinic, and isonicotinic acid].
Ann. Univ. Mariae Curie. Sklodowska. Med. 44 , 41-51, (1989)
|
|
|
Evidence for isoniazid-dependent free radical generation catalyzed by Mycobacterium tuberculosis KatG and the isoniazid-resistant mutant KatG(S315T).
Biochemistry 40(30) , 8990-6, (2001) The antitubercular agent isoniazid can be activated by Mycobacterium tuberculosis KatG using either a peroxidase compound I/II or a superoxide-dependent oxyferrous pathway. The identity of activated isoniazid is unknown, but it has been suggested that it may ... |
|
|
Design and synthesis of 2-phenoxynicotinic acid hydrazides as anti-inflammatory and analgesic agents.
Arch. Pharm. (Weinheim) 343(9) , 509-18, (2010) A series of 2-phenoxynicotinic acid hydrazides were synthesized and evaluated for their analgesic and anti-inflammatory activities. Several compounds having an unsubstituted phenyl/4-pyridyl or C-4 methoxy substituent on the terminal phenyl ring showed modera... |
|
|
Different oxidative pathways of isonicotinic acid hydrazide and its meta-isomer, nicotinic acid hydrazide.
Int. J. Biochem. 26(9) , 1081-93, (1994) 1. Superoxide was generated during the auto-oxidation of the antituberculous drug, isonicotinic acid hydrazide (INH), but not with its meta-isomer, nicotinic acid hydrazide (NH). During Fe(3+)-stimulated oxidation of INH and NH, aromatic hydroxylation occurre... |
|
|
Thermodynamic analysis of the binding of aromatic hydroxamic acid analogues to ferric horseradish peroxidase.
Biochemistry 40(46) , 13980-9, (2001) Peroxidases typically bind their reducing substrates weakly, with K(d) values in the millimolar range. The binding of benzhydroxamic acid (BHA) to ferric horseradish peroxidase isoenzyme C (HRPC) [K(d) = 2.4 microM; Schonbaum, G. R. (1973) J. Biol. Chem. 248,... |