Peroxidase-mediated oxidation of isoniazid.
H A Shoeb, B U Bowman, A C Ottolenghi, A J Merola
Index: Antimicrob. Agents Chemother. 27(3) , 399-403, (1985)
Full Text: HTML
Abstract
Oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase at the expense of H2O2 yielded reactive species which were able to reduce nitroblue tetrazolium and bleach p-nitrosodimethylaniline. Nicotinic acid hydrazide oxidation did not cause these effects. At slightly alkaline pH, oxidation of isonicotinic acid hydrazide by horseradish peroxidase proceeded at the expense of molecular O2, and the reaction was oxygen consuming. The addition of H2O2 abolished O2 consumption. Bovine liver catalase enhanced the rate of nitroblue tetrazolium reduction and decreased the maximal velocity of the reaction proportionately to catalase concentration. During oxidation of isonicotinic acid hydrazide by horseradish peroxidase-H2O2, splitting of the heme group of horseradish peroxidase took place as shown by the disappearance of the Soret and minor bands in the visible region of the spectrum.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
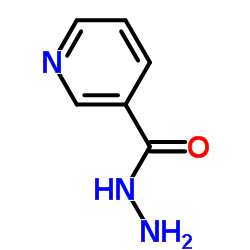 |
Nicotinohydrazide
CAS:553-53-7 |
C6H7N3O |
|
Controlled release studies of antimalarial 1, 3, 5-trisubsti...
2015-01-01 [Colloids Surf. B Biointerfaces 125 , 151-9, (2015)] |
|
Chemokine CCL17 induced by hypoxia promotes the proliferatio...
2015-01-01 [Am. J. Cancer Res. 5 , 3072-84, (2015)] |
|
Double-level "orthogonal" dynamic combinatorial libraries on...
2001-02-13 [Proc. Natl. Acad. Sci. U. S. A. 98(4) , 1347-52, (2001)] |
|
T cell-mediated induction of thymic stromal lymphopoietin in...
2014-07-01 [Clin. Exp. Allergy 44(7) , 953-64, (2014)] |
|
[Tautomerism of 2-hydrazino-4-phenylthiazole<-->4-phenylthia...
1989-01-01 [Ann. Univ. Mariae Curie. Sklodowska. Med. 44 , 41-51, (1989)] |