1,8-Naphthalic anhydride
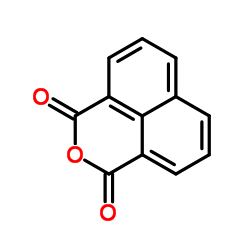
1,8-Naphthalic anhydride structure
|
Common Name | 1,8-Naphthalic anhydride | ||
|---|---|---|---|---|
| CAS Number | 81-84-5 | Molecular Weight | 198.174 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 407.5±14.0 °C at 760 mmHg | |
| Molecular Formula | C12H6O3 | Melting Point | 269 °C | |
| MSDS | Chinese USA | Flash Point | 206.8±17.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
1,8-Naphthalic anhydride antidote enhances the toxic effects of captan and thiram fungicides on Azospirillum brasilense cells.
Res. Microbiol. 142(9) , 1005-12, (1991) The effects of ten fungicides, six herbicides and four insecticides on the nitrogen-fixing bacterium Azospirillum brasilense were examined. The fungicides captan and thiram were the most toxic among the compounds tested. Cell growth and nitrogenase activity o... |
|
|
Sensitive enantiomeric separation of aliphatic and aromatic amines using aromatic anhydrides as nonchiral derivatizing agents.
J. Chromatogr. A. 666 , 485-491, (1994) New pre-column derivatizing reagents: phthalic anhydride, 3-nitrophthalic anhydride, diphenic anhydride, 1,8-naphthalic anhydride and diphenylmaleic anhydride have been developed for resolving chiral compounds having amine groups. Although all of these agents... |
|
|
Differential induction of cytochrome P450-mediated triasulfuron metabolism by naphthalic anhydride and triasulfuron.
Plant Physiol. 109(4) , 1483-90, (1995) Cytochrome P450 monooxygenases play paramount roles in the detoxification of herbicides as well as in the synthesis of lignins, flavonoids, and phenolic acids. Biochemical analysis of triasulfuron metabolism in maize (Zea mays) seedlings has demonstrated that... |
|
|
A herbicide antidote (safener) induces the activity of both the herbicide detoxifying enzyme and of a vacuolar transporter for the detoxified herbicide.
FEBS Lett. 352(2) , 219-21, (1994) In plants potentially toxic compounds are ultimately deposited in the large central vacuole. In this report we show that isolated barley mesophyll vacuoles take up the glucoside conjugate of the herbicide derivate [5-hydroxyphenyl]primisulfuron. Transport is ... |
|
|
Inosine protects against the development of diabetes in multiple-low-dose streptozotocin and nonobese diabetic mouse models of type 1 diabetes.
Mol. Med. 9(3-4) , 96-104, (2003) Inosine, a naturally occurring purine, was long considered to be an inactive metabolite of adenosine. However, recently inosine has been shown to be an immunomodulator and anti-inflammatory agent. The aim of this study was to determine whether inosine influen... |
|
|
Cloning, functional expression, and characterization of CYP709C1, the first sub-terminal hydroxylase of long chain fatty acid in plants. Induction by chemicals and methyl jasmonate.
J. Biol. Chem. 280(43) , 35881-9, (2005) We cloned and characterized CYP709C1, a new plant cytochrome P450 belonging to the P450 family, that so far has no identified function except for clustering with a fatty acid metabolizing clade of P450 enzymes. We showed here that CYP709C1 is capable of hydro... |
|
|
A colorimetric and fluorescent dual probe for specific detection of cysteine based on intramolecular nucleophilic aromatic substitution.
Analyst 137 , 5046-5050, (2012) 4-Nitro-1,8-naphthalic anhydride (NNA) was used to distinguish cysteine from homocysteine and other potentially interfering thiols through a novel sequential substitution mechanism. The discrimination involves a blue-fluorescent thioether formation via nucleo... |
|
|
Synthesis of new amonafide analogues via coupling reaction and their cytotoxic evaluation and DNA-binding studies.
Bioorg. Med. Chem. 17(2) , 804-10, (2009) A series of 5-alkylamino substituted amonafide analogues were synthesized from naphthalic anhydride by three steps including bromization, amination and CuI/proline catalyzed coupling reaction. The CuI/L-proline catalyzed coupling reaction was first applied to... |
|
|
Gas phase and bulk ultraviolet photoemission spectroscopy of 3,4,9,10-perylene-tetracarboxylic dianhydride, 1,4,5,8-naphthalene-tetracarboxylic dianhydride, and 1,8-naphthalene-dicarboxylic anhydride.
J. Chem. Phys. 131(3) , 034711, (2009) The pi-conjugated organic molecules 3,4,9,10-perylene-tetracarboxylic dianhydride, 1,4,5,8-naphthalene-tetracarboxylic dianhydride, and 1,8-naphthalene-dicarboxylic anhydride were investigated via gas phase and bulk ultraviolet photoemission spectroscopy and ... |
|
|
In vitro hydroxylation of bentazon by microsomes from naphthalic anhydride-treated corn shoots.
Biochem. Biophys. Res. Commun. 168(1) , 206-13, (1990) In vitro metabolism of the herbicide bentazon was studied in microsomal membranes isolated from 6-day-old etiolated corn shoots. Microsomes isolated from shoots of nontreated seeds did not metabolize bentazon when assayed with NADPH or peroxides. However, mic... |