Synthesis of new amonafide analogues via coupling reaction and their cytotoxic evaluation and DNA-binding studies.
Lijuan Xie, Yufang Xu, Fang Wang, Jianwen Liu, Xuhong Qian, Jingnan Cui, Lijuan Xie, Yufang Xu, Fang Wang, Jianwen Liu, Xuhong Qian, Jingnan Cui, Lijuan Xie, Yufang Xu, Fang Wang, Jianwen Liu, Xuhong Qian, Jingnan Cui
Index: Bioorg. Med. Chem. 17(2) , 804-10, (2009)
Full Text: HTML
Abstract
A series of 5-alkylamino substituted amonafide analogues were synthesized from naphthalic anhydride by three steps including bromization, amination and CuI/proline catalyzed coupling reaction. The CuI/L-proline catalyzed coupling reaction was first applied to the naphthalimide system. These new amonafide analogues showed potential anticancer activities against HeLa and P388D1 cell lines in vitro, and 4a, 4b, and 4h exhibited better activity than amonafide against HeLa cell under the same experimental conditions. More importantly, the new analogues could avoid the side effect of amonafide due to their structure, in which lacks a primary amine at the 5 position. Moreover, the DNA-binding of the analogues was also investigated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
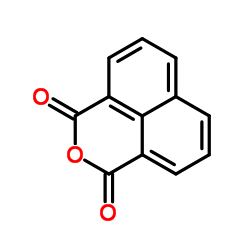 |
1,8-Naphthalic anhydride
CAS:81-84-5 |
C12H6O3 |
|
1,8-Naphthalic anhydride antidote enhances the toxic effects...
1991-01-01 [Res. Microbiol. 142(9) , 1005-12, (1991)] |
|
Sensitive enantiomeric separation of aliphatic and aromatic ...
1994-04-22 [J. Chromatogr. A. 666 , 485-491, (1994)] |
|
Differential induction of cytochrome P450-mediated triasulfu...
1995-12-01 [Plant Physiol. 109(4) , 1483-90, (1995)] |
|
A herbicide antidote (safener) induces the activity of both ...
1994-09-26 [FEBS Lett. 352(2) , 219-21, (1994)] |
|
Inosine protects against the development of diabetes in mult...
2003-01-01 [Mol. Med. 9(3-4) , 96-104, (2003)] |