Sulfamethizole
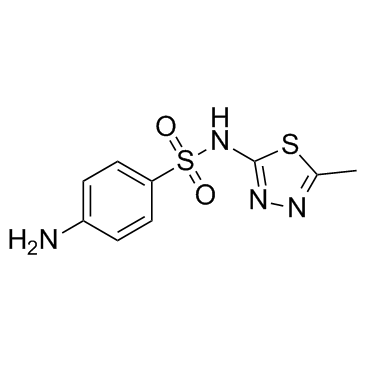
Sulfamethizole structure
|
Common Name | Sulfamethizole | ||
|---|---|---|---|---|
| CAS Number | 144-82-1 | Molecular Weight | 270.331 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 504.9±52.0 °C at 760 mmHg | |
| Molecular Formula | C9H10N4O2S2 | Melting Point | 210 °C | |
| MSDS | Chinese USA | Flash Point | 259.1±30.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental drug discovery projects toward safer medicines. In this st... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI types (hepatotoxic side effects) seen in the clinic can be tra... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals can make a significant contribution to the identification o... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predictive in vivo, in vitro, and in silico models to identify comp... |
|
|
Fast determination of 22 sulfonamides from chicken breast muscle using core-shell nanoring amino-functionalized superparamagnetic molecularly imprinted polymer followed by liquid chromatography-tandem mass spectrometry.
J. Chromatogr. A. 1345 , 17-28, (2014) A novel, simple and sensitive method was developed for the simultaneous determination of 22 sulfonamides (SAs) in chicken breast muscle by using the dispersive micro-solid-phase extraction (d-μ-SPE) procedure combined with ultra-fast liquid chromatography-tan... |
|
|
Evaluation of stationary phases packed with superficially porous particles for the analysis of pharmaceutical compounds using supercritical fluid chromatography.
J. Chromatogr. A. 1360 , 275-87, (2014) Superficially porous particles (SPP), or core shell particles, which consist of a non-porous silica core surrounded by a thin shell of porous silica, have gained popularity as a solid support for chromatography over the last decade. In the present study, five... |
|
|
Lipophilicity of acidic compounds: Impact of ion pair partitioning on drug design
Bioorg. Med. Chem. Lett. 21 , 3550-6, (2011) In drug discovery projects the ability to show a relationship between a compound's molecular structure and its pharmacokinetic, in vivo efficacy, or toxicity profile is paramount for the design of better analogues. To aid this understanding the measurement of... |
|
|
QSAR-based solubility model for drug-like compounds.
Bioorg. Med. Chem. 18 , 7078-84, (2010) Solubility plays a very important role in the selection of compounds for drug screening. In this context, a QSAR model was developed for predicting water solubility of drug-like compounds. First, a set of relevant parameters for establishing a drug-like chemi... |
|
|
Comparative chemometric modeling of cytochrome 3A4 inhibitory activity of structurally diverse compounds using stepwise MLR, FA-MLR, PLS, GFA, G/PLS and ANN techniques.
Eur. J. Med. Chem. 44 , 2913-22, (2009) Twenty-eight structurally diverse cytochrome 3A4 (CYP3A4) inhibitors have been subjected to quantitative structure-activity relationship (QSAR) studies. The analyses were performed with electronic, spatial, topological, and thermodynamic descriptors calculate... |
|
|
QSAR study on the antibacterial activity of some sulfa drugs: building blockers of Mannich bases.
Bioorg. Med. Chem. Lett. 15 , 405-11, (2005) Sulfa drugs are building blockers of several types of Mannich bases. Consequently, the antibacterial activities of sulfa drugs are reported in this paper, which will help in explaining and understanding antibacterial activities of Mannich bases. Reported QSAR... |