| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Piroxicam
CAS:36322-90-4 |
|
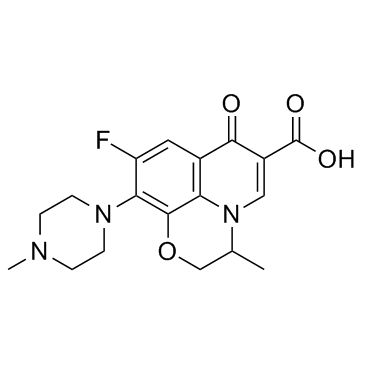 |
Ofloxacin
CAS:82419-36-1 |
|
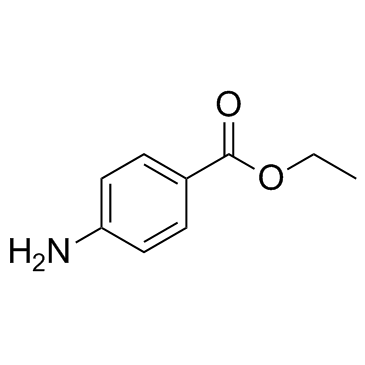 |
Benzocaine
CAS:94-09-7 |
|
 |
Salicylic acid
CAS:69-72-7 |
|
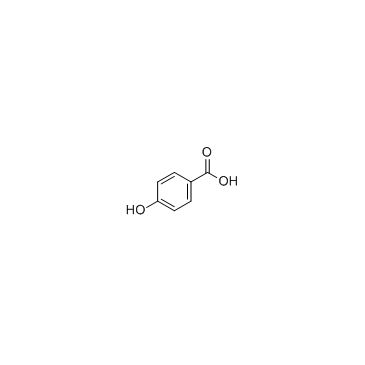 |
4-Hydroxybenzoic acid
CAS:99-96-7 |
|
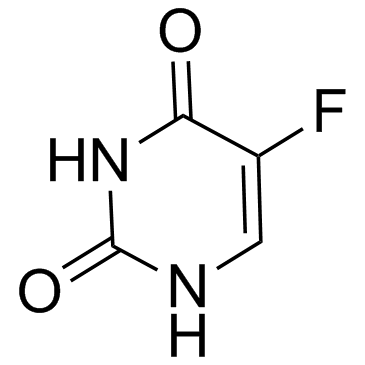 |
Fluorouracil
CAS:51-21-8 |
|
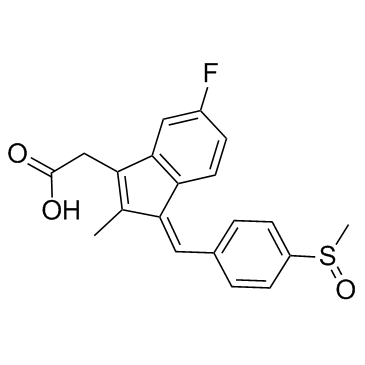 |
Sulindac
CAS:38194-50-2 |
|
 |
Famotidine
CAS:76824-35-6 |
|
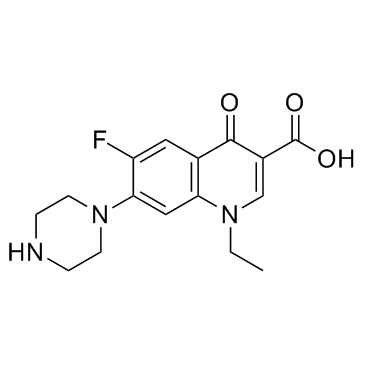 |
Norfloxacin
CAS:70458-96-7 |
|
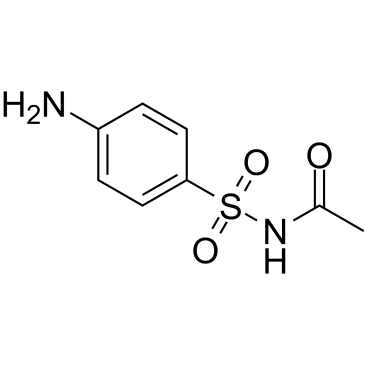 |
sulfacetamide
CAS:144-80-9 |