Salicylic acid

Salicylic acid structure
|
Common Name | Salicylic acid | ||
|---|---|---|---|---|
| CAS Number | 69-72-7 | Molecular Weight | 138.121 | |
| Density | 1.44 | Boiling Point | 211 ºC (20 mmHg) | |
| Molecular Formula | C7H6O3 | Melting Point | 158-161 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 157 ºC | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
Use of Salicylic acidSalicylic acid inhibits cyclo-oxygenase-2 (COX-2) activity independently of transcription factor (NF-κB) activation. |
| Name | salicylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Salicylic acid inhibits cyclo-oxygenase-2 (COX-2) activity independently of transcription factor (NF-κB) activation. |
|---|---|
| Related Catalog | |
| Target |
COX-2 Autophagy Mitophagy |
| In Vitro | Salicylic acid is an effective inhibitor of COX-2 activity at concentrations far below those required to inhibit NF-κB (20 mg/mL) activation. Salicylic acid inhibits prostaglandin E2 release when add together with interleukin 1β for 24 hr with an IC50 value of 5 μg/mL, an effect that is independent of NF-κB activation or COX-2 transcription or translation. Salicylic acid acutely (30 min) also causes a concentration-dependent inhibition of COX-2 activity measured in the presence of 0, 1, or 10 μM exogenous arachidonic acid. In contrast, when exogenous arachidonic acid is increased to 30 μM, Salicylic acid is a very weak inhibitor of COX-2 activity with an IC50 of >100 μg/mL. When added together with IL-1β for 24 hr, Salicylic acid causes a concentration-dependent inhibition of PGE2 release with an apparent IC50 value of approximately 5 μg/mL. The ability of Salicylic acid to directly inhibit COX-2 activity in A549 cells is tested after a 30-min exposure period, followed by the addition of different concentrations of exogenous arachidonic acid (1, 10, and 30 μM). Salicylic acid causes a concentration-dependent inhibition of COX-2 activity in the absence of added arachidonic acid or in the presence of 1 or 10 μM exogenous substrate with an apparent IC50 value of approximately 5 μg/mL. However, when the same experiments are performed using 30 μM arachidonic acid, Salicylic acid is an ineffective inhibitor of COX-2 activity, with an apparent IC50 value of more than 100 μg/mL, and achieves a maximal inhibition of less than 50%[1]. |
| In Vivo | In C57Bl/6 DIO mice, Salicylic acid decreases both fasting and postprandial plasma glucose levels. Furthermore, there is a trend to reduce plasma triglyceride levels after Salicylic acid treatment in C57Bl/6 DIO mice (P=0.059). Salicylic acid significantly reduces 11β-HSD1 mRNA in omental adipose tissue in C57Bl/6 DIO mice, with a similar trend in mesenteric adipose (P=0.057). In mesenteric adipose of C57Bl/6 DIO mice, Salicylic acid also reduces 11β-HSD1 enzyme activity[2]. |
| Kinase Assay | Human purified COX-2 are and the cofactors Glutathione (5 mM), Adrenaline (5 mM), and Hematin (1 μM) are dissolved in 50 mM Tris buffer (pH 7.5). Hematin is first dissolved in a concentrated stock of 100 mM in 1 M NaOH before being further diluted in Tris buffer. Enzyme reactions are carried out in individual wells of 96-well plates with a final reaction volume of 200 μL. Different concentrations of Salicylic acid are added to the plate, followed by the addition of 10 units of enzyme (180 μL). The plates are incubated at 37° for 30 min before Arachidonic acid (10 nM to 30 μM) is added for a further 15 min. The reaction is stopped by heating the plate to 100°C for 5 min. The 96-well plate is then centrifuged at 10,000× g for 10 min, and appropriated samples are removed and added into the radioimmunoassay[1]. |
| Cell Assay | To assess the direct effect of Salicylic acid on COX-2 activity after induction has occurred, A549 cells are first treated with IL-1β for 24 hr, and the culture medium is replaced with DMEM containing different concentrations of Salicylic acid(10, 100 and 1000 μg/mL). Cells are incubated at 37°C for 30 min. Arachidonic acid (1-30 μM) is then added for 15 min, and the medium is removed for the measurement of PGE2[1]. |
| Animal Admin | Mice[2] Adult male C57Bl/6 mice are at age 12 weeks. Diet-induced obese C57Bl/6 mice (C57Bl/6 DIO) are given 10 weeks of high-fat diet (58% fat, 12% sucrose) before treatment. Salicylic acid (120 mg/kg/day) is administered from 1 week after arriving (C57Bl/6 Lean), after 10 weeks of high-fat feeding (C57Bl/6 DIO), or after achieving target weight (HSD1KO-DIO) for 4 weeks to groups of n=8 via osmotic minipumps implant subcutaneously between the scapulae. |
| References |
| Density | 1.44 |
|---|---|
| Boiling Point | 211 ºC (20 mmHg) |
| Melting Point | 158-161 °C(lit.) |
| Molecular Formula | C7H6O3 |
| Molecular Weight | 138.121 |
| Flash Point | 157 ºC |
| Exact Mass | 138.031693 |
| PSA | 57.53000 |
| LogP | 2.06 |
| Vapour density | 4.8 (vs air) |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.616 |
| Storage condition | Store at RT. |
| Water Solubility | 1.8 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H318 |
| Precautionary Statements | P280-P301 + P312 + P330-P305 + P351 + P338 + P310 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/37/38;R41 |
| Safety Phrases | S26-S39-S37/39 |
| RIDADR | 1993.0 |
| WGK Germany | 1 |
| RTECS | VO0525000 |
| Hazard Class | 3.0 |
| HS Code | 2918211000 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2918211000 |
|---|---|
| Summary | 2918211000. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Inhibition of aberrant complement activation by a dimer of acetylsalicylic acid.
Neurobiol. Aging 36 , 2748-56, (2015) We here report synthesis for the first time of the acetyl salicylic acid dimer 5,5'-methylenebis(2-acetoxybenzoic acid) (DAS). DAS inhibits aberrant complement activation by selectively blocking facto... |
|
|
Graphene modified glassy carbon sensor for the determination of aspirin metabolites in human biological samples.
Talanta 143 , 328-34, (2015) A graphene modified glassy carbon (GR/GCE) sensor has been developed for the determination of aspirin metabolites 2,3- and 2,5-dihydroxybenzoic acids (2,3- and 2,5-DHB). The modified sensor was charac... |
|
|
Safety and biocompatibility of carbohydrate-functionalized polyanhydride nanoparticles.
AAPS J. 17(1) , 256-67, (2015) Carbohydrate functionalization of nanoparticles allows for targeting of C-type lectin receptors. This family of pattern recognition receptors expressed on innate immune cells, such as macrophages and ... |
| Verrugon |
| Acid, Salicylic |
| Duoplant |
| Freezone |
| MFCD00002439 |
| Acid, o-Hydroxybenzoic |
| SAX |
| EINECS 200-712-3 |
| Saligel |
| Hydroxybenzenecarboxylic acid |
| Salonil |
| phenol-carboxylic acid |
| Stri-Dex |
| Benzoic acid, 2-hydroxy- |
| ORTHO-HYDROXYBENZOIC ACID |
| 2-Hydroxybenzenecarboxylic acid |
| 2 Hydroxybenzoic Acid |
| Salicylic acid |
| Acid, 2-Hydroxybenzoic |
| Benzoic acid, o-hydroxy- |
| 2-hydroxy-benzoic acid |
| ortho Hydroxybenzoic Acid |
| Ionil |
| 2-hydroxybenzoic acid |
| Acid, ortho-Hydroxybenzoic |
| o-hydroxybenzoic acid |
| Duofilm |
| Rutranex |
| Keralyt |
| Lamivudine Impurity 3 |
 CAS#:90-01-7
CAS#:90-01-7 CAS#:118-91-2
CAS#:118-91-2 CAS#:88-65-3
CAS#:88-65-3 CAS#:124-38-9
CAS#:124-38-9 CAS#:100-67-4
CAS#:100-67-4 CAS#:65-85-0
CAS#:65-85-0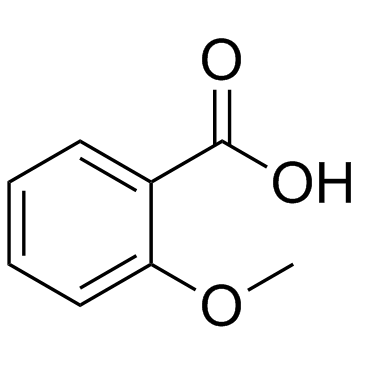 CAS#:579-75-9
CAS#:579-75-9 CAS#:14389-86-7
CAS#:14389-86-7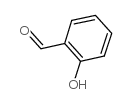 CAS#:90-02-8
CAS#:90-02-8![5-[[(3-carboxy-4-hydroxyphenyl)methylcarbamothioylamino]methyl]-2-hydroxybenzoic acid structure](https://image.chemsrc.com/caspic/421/111297-80-4.png) CAS#:111297-80-4
CAS#:111297-80-4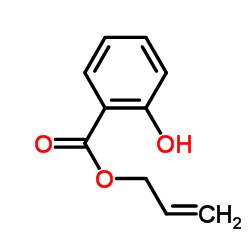 CAS#:10484-09-0
CAS#:10484-09-0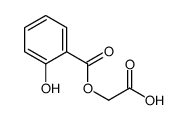 CAS#:108085-52-5
CAS#:108085-52-5 CAS#:606-45-1
CAS#:606-45-1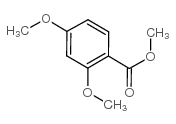 CAS#:2150-41-6
CAS#:2150-41-6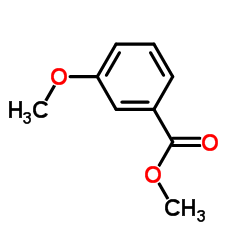 CAS#:5368-81-0
CAS#:5368-81-0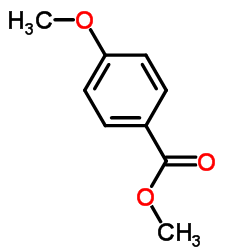 CAS#:121-98-2
CAS#:121-98-2 CAS#:2150-38-1
CAS#:2150-38-1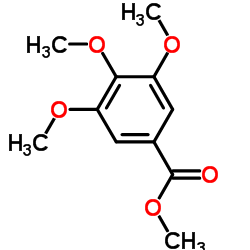 CAS#:1916-07-0
CAS#:1916-07-0 CAS#:2150-42-7
CAS#:2150-42-7
