N6-acetyl-L-lysine
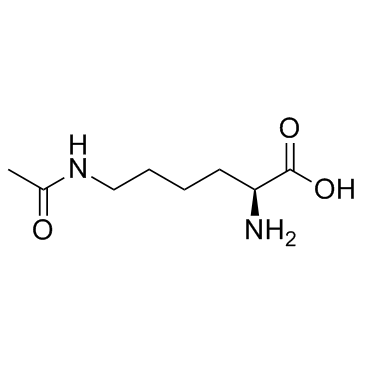
N6-acetyl-L-lysine structure
|
Common Name | N6-acetyl-L-lysine | ||
|---|---|---|---|---|
| CAS Number | 692-04-6 | Molecular Weight | 188.224 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 442.0±40.0 °C at 760 mmHg | |
| Molecular Formula | C8H16N2O3 | Melting Point | 250 °C (dec.)(lit.) | |
| MSDS | USA | Flash Point | 221.1±27.3 °C | |
|
Hypernuclear acetylation in atherosclerotic lesions and activated vascular smooth muscle cells.
Biochem. Biophys. Res. Commun. 266(2) , 417-24, (1999) Recent studies have implicated acetylation of several nuclear proteins such as histones and p53 on their epsilon-portion of lysine residues in eukaryotic transcription. Here we raised a specific polyclonal antibody against epsilon-acetylated lysine. Using the... |
|
|
Proteomic analysis of organ-specific post-translational lysine-acetylation and -methylation in mice by use of anti-acetyllysine and -methyllysine mouse monoclonal antibodies.
Proteomics 5(18) , 4653-64, (2005) Post-translational lysine-acetylation and -methylation are two major PTMs of lysine residues in proteins. Recently, we established pan-reactive anti-acetyllysine mouse mAbs, which can bind to Nepsilon-acetylated lysine residues in various contexts of amino ac... |
|
|
Purification and characterization of a novel aminoacylase from Streptomyces mobaraensis.
Biosci. Biotechnol. Biochem. 69 , 1914-1922, (2005) A novel aminoacylase was purified to homogeneity from culture broth of Streptomyces mobaraensis, as evidenced by SDS-polyacrylamide gel electrophoresis (PAGE). The enzyme was a monomer with an approximate molecular mass of 100 kDa. The purified enzyme was inh... |
|
|
Solution structure and acetyl-lysine binding activity of the GCN5 bromodomain.
J. Mol. Biol. 304(3) , 355-70, (2000) The solution structure of the bromodomain from the human transcriptional coactivator GCN5 has been determined using NMR methods. The structure has a left-handed four-helix bundle topology, with two short additional helices in a long connecting loop. A hydroph... |
|
|
Quantitative analysis of histone modifications: formaldehyde is a source of pathological n(6)-formyllysine that is refractory to histone deacetylases.
PLoS Genet. 9(2) , e1003328, (2013) Aberrant protein modifications play an important role in the pathophysiology of many human diseases, in terms of both dysfunction of physiological modifications and the formation of pathological modifications by reaction of proteins with endogenous electrophi... |
|
|
Characterization of a novel enzyme, N6-acetyl-L-lysine: 2-oxoglutarate aminotransferase, which catalyses the second step of lysine catabolism in Candida maltosa.
Antonie van Leeuwenhoek 62(4) , 285-90, (1992) A novel aminotransferase catalyzing the second step of lysine catabolism, the oxidative transamination of the alpha-group of N6-acetyllysine, was identified and characterized in the yeast Candida maltosa. The enzyme was strongly induced in cells grown on L-ly... |
|
|
A case of hyperlysinemia: biochemical and clinical observations.
Pediatrics 39(4) , 546-54, (1967)
|
|
|
Chemical characterization of a protein-4-hydroxy-2-nonenal cross-link: immunochemical detection in mitochondria exposed to oxidative stress.
Arch. Biochem. Biophys. 328(1) , 158-64, (1996) We have previously shown that incubation of the model protein glucose-6-phosphate dehydrogenase (Glu-6-PDH) from the bacterium Leuconostoc mesenteroides with 4-hydroxy-2-nonenal (HNE), a major product of lipid peroxidation, results in the formation of cross-l... |
|
|
p-Hydroxyphenylacetaldehyde, the major product of L-tyrosine oxidation by the myeloperoxidase-H2O2-chloride system of phagocytes, covalently modifies epsilon-amino groups of protein lysine residues.
J. Biol. Chem. 272(27) , 16990-8, (1997) Activated human phagocytes employ the myeloperoxidase-H2O2-Cl- system to convert L-tyrosine to p-hydroxyphenylacetaldehyde (pHA). We have explored the possibility that pHA covalently reacts with proteins to form Schiff base adducts, which may play a role in m... |
|
|
A convenient method for genetic incorporation of multiple noncanonical amino acids into one protein in Escherichia coli.
Mol. Biosyst. 6(4) , 683-6, (2010) By overexpressing the C-terminal domain of the ribosomal protein L11 to decrease release factor 1-mediated termination of protein translation, enhanced amber suppression is achieved in E. coli. This enhanced amber suppression efficiency allows the genetic inc... |