Chemical characterization of a protein-4-hydroxy-2-nonenal cross-link: immunochemical detection in mitochondria exposed to oxidative stress.
J A Cohn, L Tsai, B Friguet, L I Szweda
Index: Arch. Biochem. Biophys. 328(1) , 158-64, (1996)
Full Text: HTML
Abstract
We have previously shown that incubation of the model protein glucose-6-phosphate dehydrogenase (Glu-6-PDH) from the bacterium Leuconostoc mesenteroides with 4-hydroxy-2-nonenal (HNE), a major product of lipid peroxidation, results in the formation of cross-linked protein. HNE-modified protein is resistant to proteolytic degradation and acts as an inhibitor of the multicatalytic proteinase. It was therefore important to establish the chemistry of the cross-linking reaction. The formation of cross-linked Glu-6-PDH is associated with the nearly exclusive loss of lysine residues. For this reason the reaction of N-acetyllysine with HNE has been investigated. The epsilon-amino group of lysine reacts with the double bond (C3) and the carbonyl (C1) functions of HNE via Michael addition and Schiff base formation resulting in the production of a 2:1 amino acid-HNE cross-link. Chromatographic detection of this adduct in the acid hydrolysate of HNE-treated Glu-6-PDH reveals that this chemistry is responsible for the formation of cross-linked protein. Antibody to the reduced form of the 2:1 lysine-HNE adduct was prepared. The antibody was used to demonstrate that exposure of isolated liver mitochondria to oxidative stress led to the formation of intra- and intermolecular protein-HNE cross-links. The results of the present study indicate that modifications to protein by lipid peroxidation products may be physiologically relevant and could contribute to the disease- and age-related buildup of damaged protein.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
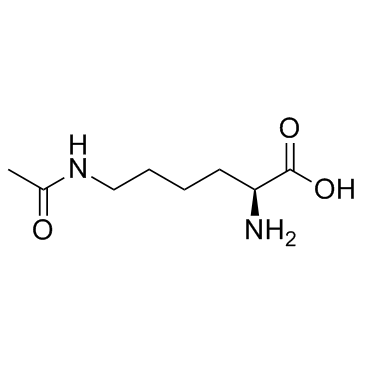 |
N6-acetyl-L-lysine
CAS:692-04-6 |
C8H16N2O3 |
|
Hypernuclear acetylation in atherosclerotic lesions and acti...
1999-12-20 [Biochem. Biophys. Res. Commun. 266(2) , 417-24, (1999)] |
|
Proteomic analysis of organ-specific post-translational lysi...
2005-12-01 [Proteomics 5(18) , 4653-64, (2005)] |
|
Purification and characterization of a novel aminoacylase fr...
2005-10-01 [Biosci. Biotechnol. Biochem. 69 , 1914-1922, (2005)] |
|
Solution structure and acetyl-lysine binding activity of the...
2000-12-01 [J. Mol. Biol. 304(3) , 355-70, (2000)] |
|
Quantitative analysis of histone modifications: formaldehyde...
2013-01-01 [PLoS Genet. 9(2) , e1003328, (2013)] |