Characterization of a novel enzyme, N6-acetyl-L-lysine: 2-oxoglutarate aminotransferase, which catalyses the second step of lysine catabolism in Candida maltosa.
H Schmidt, R Bode
Index: Antonie van Leeuwenhoek 62(4) , 285-90, (1992)
Full Text: HTML
Abstract
A novel aminotransferase catalyzing the second step of lysine catabolism, the oxidative transamination of the alpha-group of N6-acetyllysine, was identified and characterized in the yeast Candida maltosa. The enzyme was strongly induced in cells grown on L-lysine as sole carbon source. Its activity was specific for both N6-acetyllysine and 2-oxoglutarate. The Km values were 14 mM for the donor, 4 mM for the acceptor and 1.7 microM for pyridoxal-5-phosphate. The enzyme had a maximum activity at pH 8.1 and 32 degrees C. Its molecular mass estimated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis was 55 kDa. Since the native molecular mass determined by gel filtration was 120 kDa, the enzyme is probably a homodimer.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
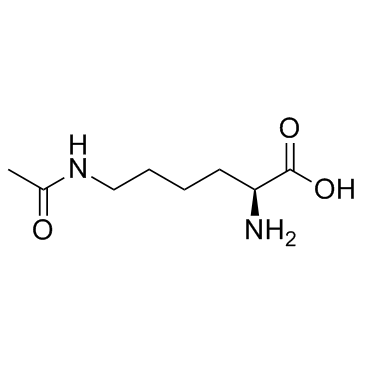 |
N6-acetyl-L-lysine
CAS:692-04-6 |
C8H16N2O3 |
|
Hypernuclear acetylation in atherosclerotic lesions and acti...
1999-12-20 [Biochem. Biophys. Res. Commun. 266(2) , 417-24, (1999)] |
|
Proteomic analysis of organ-specific post-translational lysi...
2005-12-01 [Proteomics 5(18) , 4653-64, (2005)] |
|
Purification and characterization of a novel aminoacylase fr...
2005-10-01 [Biosci. Biotechnol. Biochem. 69 , 1914-1922, (2005)] |
|
Solution structure and acetyl-lysine binding activity of the...
2000-12-01 [J. Mol. Biol. 304(3) , 355-70, (2000)] |
|
Quantitative analysis of histone modifications: formaldehyde...
2013-01-01 [PLoS Genet. 9(2) , e1003328, (2013)] |