Diethylamine
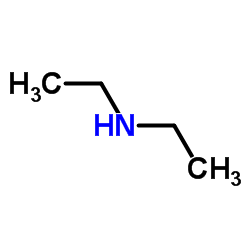
Diethylamine structure
|
Common Name | Diethylamine | ||
|---|---|---|---|---|
| CAS Number | 109-89-7 | Molecular Weight | 73.137 | |
| Density | 0.7±0.1 g/cm3 | Boiling Point | 57.3±8.0 °C at 760 mmHg | |
| Molecular Formula | C4H11N | Melting Point | -50 °C | |
| MSDS | Chinese USA | Flash Point | -28.9±0.0 °C | |
| Symbol |



GHS02, GHS05, GHS06 |
Signal Word | Danger | |
|
Controlled Endolysosomal Release of Agents by pH-responsive Polymer Blend Particles.
Pharm. Res. 32 , 2280-91, (2015) A key step of delivering extracellular agents to its intracellular target is to escape from endosomal/lysosomal compartments, while minimizing the release of digestive enzymes that may compromise cellular functions. In this study, we examined the intracellula... |
|
|
Convenient QSAR model for predicting the complexation of structurally diverse compounds with β-cyclodextrins
Bioorg. Med. Chem. 17 , 896-904, (2009) This paper reports a QSAR study for predicting the complexation of a large and heterogeneous variety of substances (233 organic compounds) with beta-cyclodextrins (beta-CDs). Several different theoretical molecular descriptors, calculated solely from the mole... |
|
|
Magnetic high throughput screening system for the development of nano-sized molecularly imprinted polymers for controlled delivery of curcumin.
Analyst 140(9) , 3113-20, (2015) Curcumin is a versatile anti-inflammatory and anti-cancer agent known for its low bioavailability, which could be improved by developing materials capable of binding and releasing drug in a controlled fashion. The present study describes the preparation of ma... |
|
|
Exploring the enantioseparation of amino-naphthol analogues by supercritical fluid chromatography.
J. Chromatogr. A. 1387 , 123-33, (2015) The direct separation of the enantiomers of 1-(α-aminoarylmethyl)-2-naphthol, 1-(α-aminoalkyl)-2-naphthol, 2-(α-aminoarylmethyl)-1-naphthol analogues and 2-(1-amino-2-methylpropyl)-1-naphthol) was investigated in supercritical fluid chromatography. Five comme... |
|
|
Investigation of the structure-selectivity relationships and van't Hoff analysis of chromatographic stereoisomer separations of unusual isoxazoline-fused 2-aminocyclopentanecarboxylic acids on Cinchona alkaloid-based chiral stationary phases.
J. Chromatogr. A. 1384 , 67-75, (2015) The enantiomers of four unusual, rather rigid isoxazoline-fused 2-aminocyclopentanecarboxylic acids were directly separated on a quinine- or a quinidine-based zwitterionic ion-exchanger as chiral selector. The effects of the mobile phase composition, the stru... |
|
|
Validation of a rapid method of analysis using ultrahigh-performance liquid chromatography - tandem mass spectrometry for nitrogen-rich adulterants in nutritional food ingredients.
J. Chromatogr. A. 1373 , 106-13, (2014) A method for the rapid quantification of 9 potential nitrogen-rich economic adulterants (dicyandiamide, urea, biuret, cyromazine, amidinourea, ammeline, amidinourea, melamine, and cyanuric acid) in five milk and soy derived nutritional ingredients, i.e. whole... |
|
|
Structural and temperature effects on enantiomer separations of bicyclo[2.2.2]octane-based 3-amino-2-carboxylic acids on cinchona alkaloid-based zwitterionic chiral stationary phases.
J. Pharm. Biomed. Anal. 98 , 130-9, (2014) Procedures for the direct high-performance liquid chromatographic enantiomer separation of four bicyclo[2.2.2]octane-based 3-amino-2-carboxylic acids were developed in polar-ionic mode on zwitterionic chiral stationary phases (CSPs) based on cinchonane alkalo... |
|
|
A novel UV degradation product of Ebastine: isolation and characterization using Q-TOF, NMR, IR and computational chemistry.
J. Pharm. Biomed. Anal. 107 , 488-94, (2015) Forced degradation of Ebastine (1-(4-(1,1-dimethylethyl)phenyl)-4-(4-(diphenylmethoxy) piperidin-1-yl)butan-1-one) drug substance in ultraviolet light condition resulted into an unknown significant degradation product. This degradation product was analyzed us... |
|
|
Direct high-performance liquid chromatographic enantioseparation of secondary amino acids on Cinchona alkaloid-based chiral zwitterionic stationary phases. Unusual temperature behavior.
J. Chromatogr. A. 1363 , 169-77, (2014) Two chiral stationary phases containing a quinine- or a quinidine-based zwitterionic ion-exchanger as chiral selector were applied for the enantioseparation of 27 unusual cyclic secondary α-amino acids. The effects of the nature and concentration of the bulk ... |
|
|
Chiral liquid chromatography-tandem mass spectrometry assay to determine that dexpramipexole is not converted to pramipexole in vivo after administered in humans.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 971 , 133-40, (2014) Dexpramipexole (DEX) was being investigated in clinical studies for the treatment of amyotrophic lateral sclerosis (ALS). To monitor the potential chiral interconversion of dexpramipexole to pramipexole (PPX) in vivo, a highly sensitive and selective chiral L... |