| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
acetic acid
CAS:64-19-7 |
|
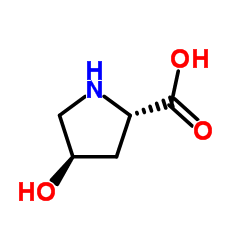 |
L-Hydroxyproline
CAS:3398-22-9 |
|
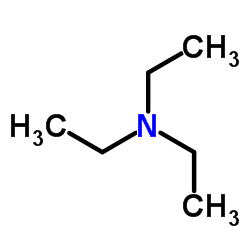 |
Triethylamine
CAS:121-44-8 |
|
 |
Ammonia
CAS:7664-41-7 |
|
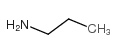 |
Propylamine
CAS:107-10-8 |
|
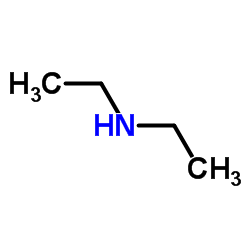 |
Diethylamine
CAS:109-89-7 |
|
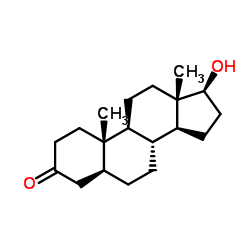 |
Stanolone
CAS:521-18-6 |