6-bromoindole
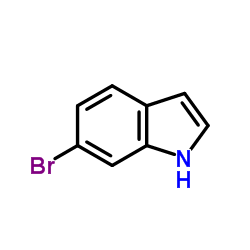
6-bromoindole structure
|
Common Name | 6-bromoindole | ||
|---|---|---|---|---|
| CAS Number | 52415-29-9 | Molecular Weight | 196.04 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 316.9±15.0 °C at 760 mmHg | |
| Molecular Formula | C8H6BrN | Melting Point | 92-96 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 145.5±20.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Indoline derivatives as 5-HT(2C) receptor agonists.
Bioorg. Med. Chem. Lett. 14 , 2367-2370, (2004) A series of 1-(1-indolinyl)-2-propylamines was synthesised and evaluated as 5-HT(2C) receptor agonists for the treatment of obesity. The general methods of synthesis of the precursor indoles are described. The functional efficacy and radioligand binding data ... |
|
|
Synthesis and biological evaluation of achiral indole-substituted titanocene dichloride derivatives.
Int J Med Chem 2012 , 905981, (2015) Six new titanocene compounds have been isolated and characterised. These compounds were synthesised from their fulvene precursors using Super Hydride (LiBEt3H) followed by transmetallation with titanium tetrachloride to yield the corresponding titanocene dich... |
|
|
Enantioselective total syntheses of (+)-arborescidine A, (-)-arborescidine B, and (-)-arborescidine C.
J. Org. Chem. 69 , 1283-1289, (2004) Described are the first enantioselective total syntheses of (+)-arborescidine A ((+)-1), (-)-arborescidine B ((-)-2), and (-)-arborescidine C ((-)-3), via routes that proceeded in five steps and 50% overall yield, eight steps and 61% overall yield, and nine s... |
|
|
Total synthesis of (+/-)-perophoramidine.
J. Am. Chem. Soc. 126 , 5068-5069, (2004) The first total synthesis of racemic perophoramidine is described. The key step features the highly stereoselective introduction of the vicinial quaternary centers via base-promoted carbon-carbon bond formation between a 3-alkylindole and a 3-bromo-3-alkylind... |
|
|
A facile synthesis of Tyrian purple based on a biosynthetic pathway. Tanoue Y, et al.
Fish. Sci. (Tokyo, Jpn.) 67(4) , 726-729, (2001)
|
|
|
Efficient synthesis of 5-and 6-tributylstannylindoles and their reactivity with acid chlorides in the Stille coupling reaction. Cherry K, et al.
Tetrahedron Lett. 48(33) , 5751-5753, (2007)
|
|
|
Synthesis of N-protected Nortopsentins B and D. Moody CJ and Roffey JRA.
ARKIVOC 1 , 393-401, (2000)
|
|
|
Palladium-catalyzed carbonylation of haloindoles: No need for protecting groups. Kumar K, et al.
Org. Lett. 6(1) , 7-10, (2004)
|