6-bromoindole
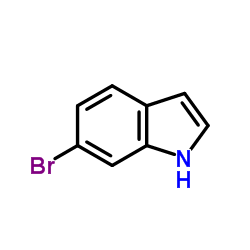
6-bromoindole structure
|
Common Name | 6-bromoindole | ||
|---|---|---|---|---|
| CAS Number | 52415-29-9 | Molecular Weight | 196.04 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 316.9±15.0 °C at 760 mmHg | |
| Molecular Formula | C8H6BrN | Melting Point | 92-96 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 145.5±20.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 6-bromoindole6-Bromoindole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 6-bromo-1H-indole |
|---|---|
| Synonym | More Synonyms |
| Description | 6-Bromoindole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 6-溴吲哚试剂 |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 316.9±15.0 °C at 760 mmHg |
| Melting Point | 92-96 °C(lit.) |
| Molecular Formula | C8H6BrN |
| Molecular Weight | 196.04 |
| Flash Point | 145.5±20.4 °C |
| Exact Mass | 194.968353 |
| PSA | 15.79000 |
| LogP | 2.91 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.712 |
| InChIKey | MAWGHOPSCKCTPA-UHFFFAOYSA-N |
| SMILES | Brc1ccc2cc[nH]c2c1 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2942000000 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Indoline derivatives as 5-HT(2C) receptor agonists.
Bioorg. Med. Chem. Lett. 14 , 2367-2370, (2004) A series of 1-(1-indolinyl)-2-propylamines was synthesised and evaluated as 5-HT(2C) receptor agonists for the treatment of obesity. The general methods of synthesis of the precursor indoles are descr... |
|
|
Synthesis and biological evaluation of achiral indole-substituted titanocene dichloride derivatives.
Int J Med Chem 2012 , 905981, (2015) Six new titanocene compounds have been isolated and characterised. These compounds were synthesised from their fulvene precursors using Super Hydride (LiBEt3H) followed by transmetallation with titani... |
|
|
Enantioselective total syntheses of (+)-arborescidine A, (-)-arborescidine B, and (-)-arborescidine C.
J. Org. Chem. 69 , 1283-1289, (2004) Described are the first enantioselective total syntheses of (+)-arborescidine A ((+)-1), (-)-arborescidine B ((-)-2), and (-)-arborescidine C ((-)-3), via routes that proceeded in five steps and 50% o... |
| 6-brom-1H-indole |
| 6-BROMINDOL |
| 6-Bromoindole |
| bromoindole-6 |
| 1H-Indole, 6-bromo- |
| 6-Bromo-1H-indole |
| 6-bromo-1 H-indole |
| MFCD00238550 |
 CAS#:16732-65-3
CAS#:16732-65-3 CAS#:108061-73-0
CAS#:108061-73-0 CAS#:881-50-5
CAS#:881-50-5 CAS#:103-88-8
CAS#:103-88-8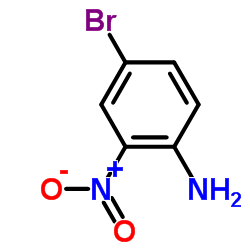 CAS#:875-51-4
CAS#:875-51-4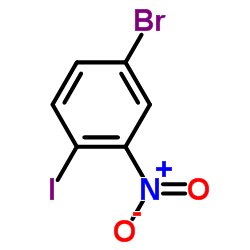 CAS#:112671-42-8
CAS#:112671-42-8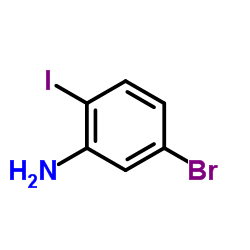 CAS#:64085-52-5
CAS#:64085-52-5 CAS#:112671-48-4
CAS#:112671-48-4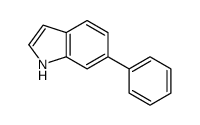 CAS#:106851-31-4
CAS#:106851-31-4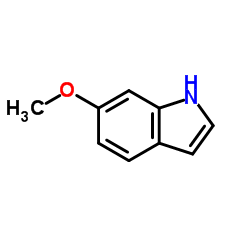 CAS#:3189-13-7
CAS#:3189-13-7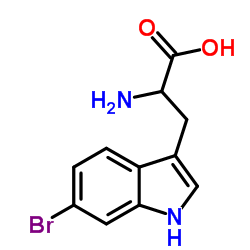 CAS#:33599-61-0
CAS#:33599-61-0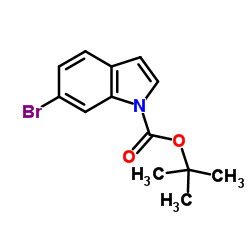 CAS#:147621-26-9
CAS#:147621-26-9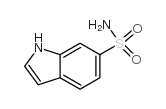 CAS#:145951-26-4
CAS#:145951-26-4![6-Bromo-1-[(4-methylphenyl)sulfonyl]-1H-indole structure](https://image.chemsrc.com/caspic/461/189265-99-4.png) CAS#:189265-99-4
CAS#:189265-99-4 CAS#:184637-11-4
CAS#:184637-11-4 CAS#:202584-22-3
CAS#:202584-22-3 CAS#:301856-44-0
CAS#:301856-44-0 CAS#:884507-19-1
CAS#:884507-19-1
