Enantioselective total syntheses of (+)-arborescidine A, (-)-arborescidine B, and (-)-arborescidine C.
Leonardo S Santos, Ronaldo A Pilli, Viresh H Rawal
Index: J. Org. Chem. 69 , 1283-1289, (2004)
Full Text: HTML
Abstract
Described are the first enantioselective total syntheses of (+)-arborescidine A ((+)-1), (-)-arborescidine B ((-)-2), and (-)-arborescidine C ((-)-3), via routes that proceeded in five steps and 50% overall yield, eight steps and 61% overall yield, and nine steps and 51% overall yield, respectively, from 6-bromotryptamine (7). The syntheses feature the use of the Noyori catalytic asymmetric hydrogen-transfer reaction to introduce chirality in dihydro-beta-carbolines 6 and 8. On the basis of an ample precedent from Noyori's work, the reduction produces dihydro-beta-carbolines, and ultimately the natural products, possessing the R absolute configuration. The synthetic arborescidines displayed optical rotations that were opposite in sign those of the natural products, thereby supporting the S configuration for natural arborescidines A (1) and B (2) and the (3S,17S) configuration for natural arborescidine C (3). Our results are in agreement with the initial stereochemical assignment by Païs and co-workers, and are counter to their recently revised assignment.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
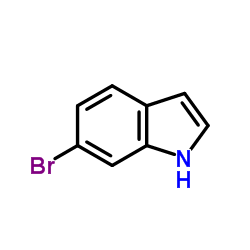 |
6-bromoindole
CAS:52415-29-9 |
C8H6BrN |
|
Indoline derivatives as 5-HT(2C) receptor agonists.
2004-05-03 [Bioorg. Med. Chem. Lett. 14 , 2367-2370, (2004)] |
|
Synthesis and biological evaluation of achiral indole-substi...
2012-01-01 [Int J Med Chem 2012 , 905981, (2015)] |
|
Total synthesis of (+/-)-perophoramidine.
2004-04-28 [J. Am. Chem. Soc. 126 , 5068-5069, (2004)] |
|
A facile synthesis of Tyrian purple based on a biosynthetic ...
[Fish. Sci. (Tokyo, Jpn.) 67(4) , 726-729, (2001)] |
|
Efficient synthesis of 5-and 6-tributylstannylindoles and th...
[Tetrahedron Lett. 48(33) , 5751-5753, (2007)] |